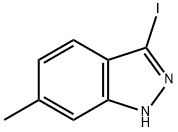
3-IODO-6-METHYL (1H)INDAZOLE synthesis
- Product Name:3-IODO-6-METHYL (1H)INDAZOLE
- CAS Number:885518-96-7
- Molecular formula:C8H7IN2
- Molecular Weight:258.06
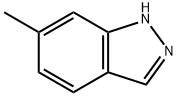
698-24-8
112 suppliers
$19.00/250mg

885518-96-7
45 suppliers
$64.00/250mg
Yield:885518-96-7 92.19%
Reaction Conditions:
with N-iodo-succinimide in N,N-dimethyl-formamide at 25; for 1 h;
Steps:
16 3-iodo-6-methyl-1H-indazole
To a solution of 6-methyl-1H-indazole (0.5 g, 3.78 mmol, 1 eq) in DMF (5 mL) was added NIS (1.28 g, 5.67 mmol, 1.5 eq) and the mixture was stirred at 25 °C for 1 hr. TLC (PE: EtOAc =4:1, SM Rf=0.40, TM Rf=0.58) showed that the reaction was complete. The reaction was poured into~10 mL water and extracted with EtOAc (3x10 mL), washed with brine (3x10 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography (SiO2, PE:EtOAc=10:1 to 1/1) to afford the title compound 3-iodo-6-methyl-1H-indazole (0.9 g, 3.49 mmol, 92.19% yield) as a white solid.
References:
WO2021/262596,2021,A1 Location in patent:Paragraph 0790