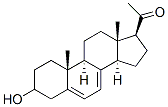
3-hydroxy-5,7-pregnadien-20-one synthesis
- Product Name:3-hydroxy-5,7-pregnadien-20-one
- CAS Number:81968-78-7
- Molecular formula:C21H30O2
- Molecular Weight:314.46
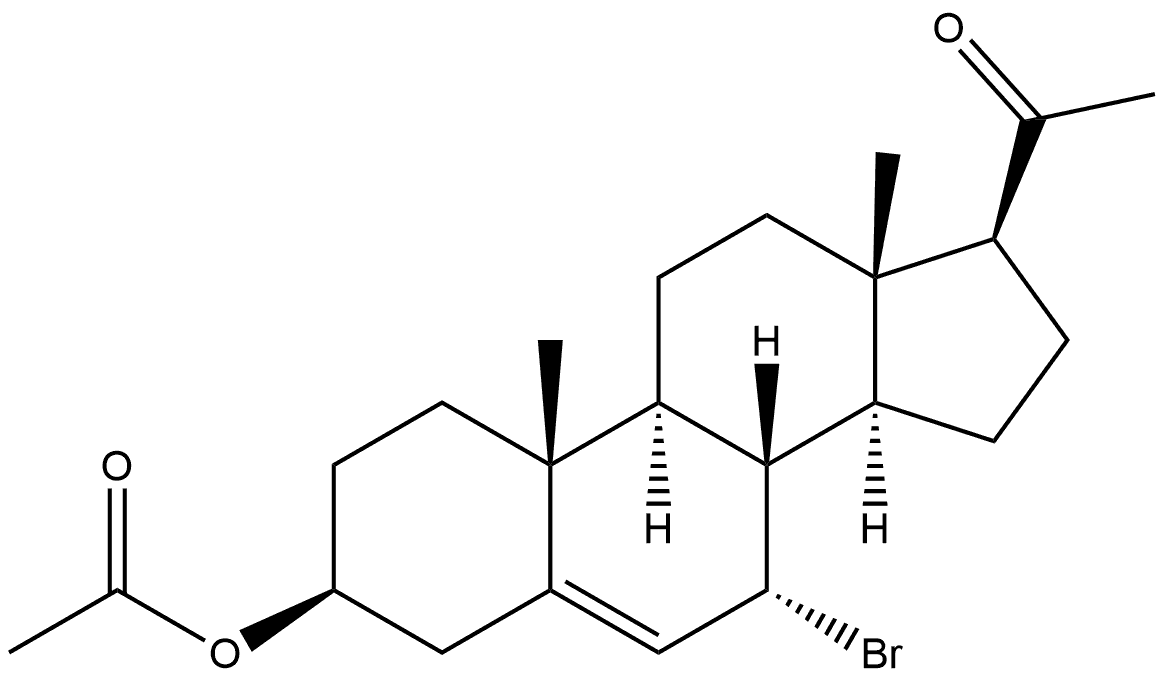
105186-40-1
0 suppliers
inquiry

81968-78-7
13 suppliers
inquiry
Yield:81968-78-7 94.3%
Reaction Conditions:
Stage #1: 3β-acetoxy-7α-bromo-pregn-5-en-20-onewith tetrabutyl ammonium fluoride in tetrahydrofuran at 0 - 25; for 3.5 h;Molecular sieve;
Stage #2: with potassium carbonate in tetrahydrofuran;methanol at 25; for 2.5 h;
Steps:
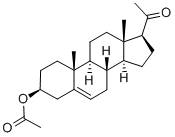
28 3β-Pregna-5,7-dienol-20-one
Example 28 3β-Pregna-5,7-dienol-20-one A solution of tetra-n-butylammonium fluoride in THF (1.0 M, 20 mL, 20 mmol), which had been predried over activated molecular sieves, was stirred at 0° C., under nitrogen and 3β,7α-bromopregn-5-enol-20-one acetate (2.914 g, 6.663 mmol) was added in one portion. After 3 hours, the ice-bath was removed, and the reaction mixture was stirred at 25° C. for 30 minutes, and then methanol (20 mL) and potassium carbonate (3.454 g, 25 mmol) were added, and the mixture was stirred vigourously at 25° C. Within an hour the mixture had become a thick beige slurry, and after 2.5 hours the reaction mixture was recooled on an ice-bath with stirring, and cold water (125 mL) was added over 5 minutes. After 30 minutes, the reaction mixture was Buchner filtered, and the residue was rinsed with cold water (2*25 mL). The residue was dried in a vacuum oven at 50° C. for 2 hours, and air dried for 48 hours to give 3β-pregna-5,7-dienol-20-one (1.976 g, 94.3%) as a pale yellowish-beige solid. Nmr analysis shows purity in the 95-6% range. 1H NMR (CDCl3 500 MHz) δ: 0.608 (3H, s), 0.966 (3H, s), 1.344 (1H, brt (J=11.5 Hz), 1.47-1.63 (5H, m), 1.67-1.87 (3H, m), 1.90-1.96 (2H, m), 2.02-2.08 (2H, m), 2.13-2.36 (3H, m), 2.177 (3H, s), 2.509 (1H, brd, J=14.5 Hz), 2.654 (1H, t, J=9.2 Hz), 3.669 (H, septet, J=5.5 Hz), 5.453 (1H, narrow m), 5.611 (1H, narrow m).
References:
US2008/171728,2008,A1 Location in patent:Page/Page column 57
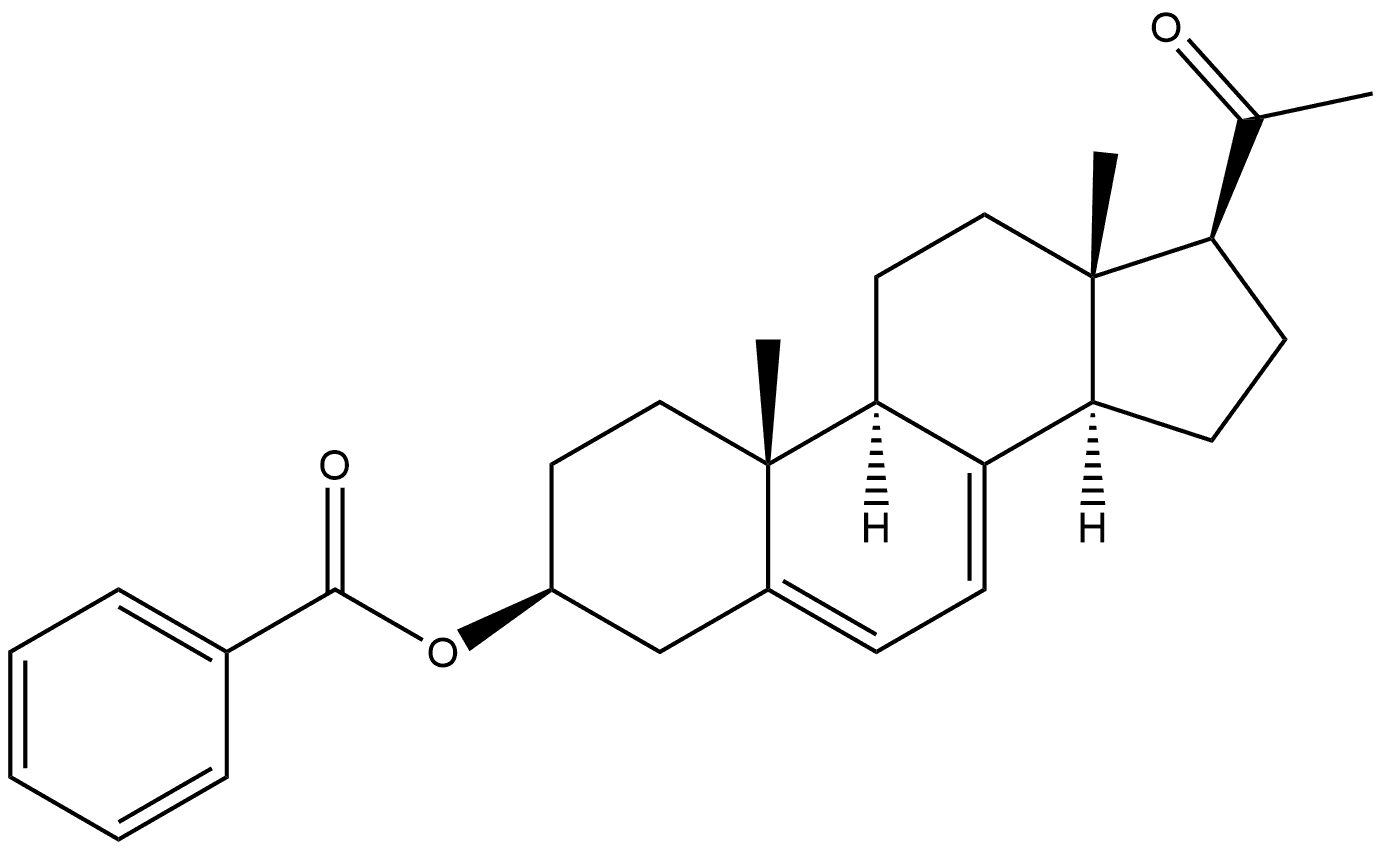
1778-02-5
264 suppliers
$12.00/1mg

81968-78-7
13 suppliers
inquiry
116028-16-1
0 suppliers
inquiry

81968-78-7
13 suppliers
inquiry
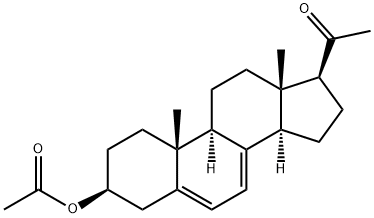
28319-79-1
1 suppliers
inquiry

81968-78-7
13 suppliers
inquiry
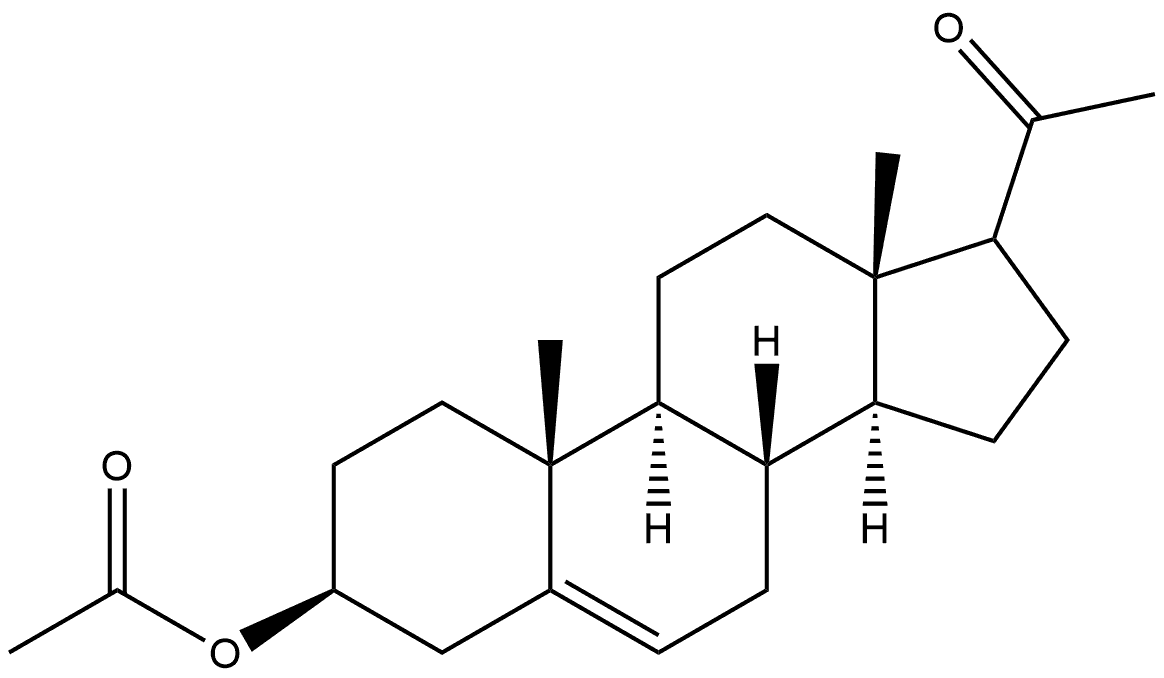
1093959-59-1
0 suppliers
inquiry

81968-78-7
13 suppliers
inquiry