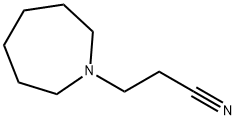
3-HEXAMETHYLENEIMINOPROPIONITRILE synthesis
- Product Name:3-HEXAMETHYLENEIMINOPROPIONITRILE
- CAS Number:937-51-9
- Molecular formula:C9H16N2
- Molecular Weight:152.24
Yield:937-51-9 88%
Reaction Conditions:
at 20; for 24 h;
Steps:
1 2.1
Synthesis of 3-Azepan-1-yl-propionitrile
To a solution of Azepane (2 g, 20.16 mmol), acrylonitrile was added and stirred at room temperature for 24 h.
The reaction mixture was poured into water and the organic layer was extracted with dichloromethane (3 * 10 mL).
The combined organic layer was evaporated using a rotary evaporator to obtain 3-Azepan-1-yl-propionitrile as a pale yellow liquid.
Yield = 2.7 g (88%).
1H NMR (DMSO,d6): δ: 1.21 (m, 4H), 1.40 (m, 4H), 2.41 (m, 4H), 2.55 (t, 2H), 2.80 (t, 2H).
13C NMR, DMSO (d6): δ: 17.2, 26.4, 29.6, 49.4, 117.2. CHN elemental analysis: Calculated: C; 71.01, H; 10.59, N; 18.40. Experimental: C; 69.98, H; 10.66, N; 18.34.
References:
Lethesh, Kallidanthiyil Chellappan;Shah, Syed Nasir;Ayodele, Olumide Bolarinwa;Mutalib, M.I. Abdul;Uemura, Yoshimitsu [Journal of Molecular Liquids,2016,vol. 221,p. 1140 - 1144]