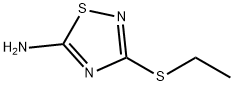
3-ETHYLSULFANYL-[1,2,4]THIADIAZOL-5-YLAMINE synthesis
- Product Name:3-ETHYLSULFANYL-[1,2,4]THIADIAZOL-5-YLAMINE
- CAS Number:6913-14-0
- Molecular formula:C4H7N3S2
- Molecular Weight:161.25
Yield:6913-14-0 77.9%
Reaction Conditions:
with bromine;triethylamine in methanol at 20; for 2 h;
Steps:
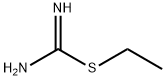
8.1 The first step: 3-(Ethylthio)-1,2,4-thiadiazol-5-amine (8B)
Sodium thiocyanate (3.86 g, 47.57 mmol) was dissolved in methanol (40 mL), then carboxamidinoethyl sulfide 8A (3.7 g, 18.29 mmol) and triethylamine (3.06 mL, 21.95 were added with stirring at 0° C. mmol). The mixture was added dropwise triethylamine (2.55 mL, 18.29 mmol) and liquid bromine (5.85 g, 36.59 mmol) simultaneously with stirring at 0°C. After 2 hours of reaction at room temperature, the reaction solution was concentrated, and the crude product was separated and purified by silica gel column chromatography (ethyl acetate/petroleum ether (v/v)=0-30%) to obtain a yellow solid compound 3-(ethylthio)-1, 2,4-Thiadiazol-5-amine 8B (2.3 g, 77.9%).
References:
WO2022/117075,2022,A1 Location in patent:Page/Page column 51