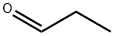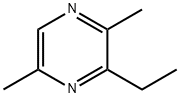
3-Ethyl-2,5-diMethylpyrazine synthesis
- Product Name:3-Ethyl-2,5-diMethylpyrazine
- CAS Number:13360-65-1
- Molecular formula:C8H12N2
- Molecular Weight:136.19
Yield:13360-65-1 90.88%
Reaction Conditions:
with ferrous(II) sulfate heptahydrate;sulfuric acid;dihydrogen peroxide in water at 50 - 60; for 6 h;
Steps:
1 Embodiment 1
1), in 125 ml in the reaction bottle, by adding 1.08g (10mmol) 2, 5-dimethyl-pyrazine, 1.13g (4mol) FeSO4· 7H2O, by adding 30 ml of water (i.e., 3 ml/1mmol), stirring in the ice bath, then measure 0.1mol (6 ml) 98% concentrated sulfuric acid to constant pressure dropping funnel slowly dropping, the temperature change in the process of dropping the attention, to maintain the temperature of the reaction bottle too high (i.e., to control the temperature to 60 °C the following can be). After concentrated sulfuric acid instillment , on a rectifier 22mmol (7.5 ml) hydrogen peroxide, PV drop instillment , and to continue the reaction in the ice bath, dripping at any time in the process of observing reaction bottle temperature change (i.e., to control the temperature to 60 °C the following can be). After dripping adding 240uL (3mmol) is propionaldehyde, slowly elevated temperature so as to control the temperature to 50-60°C, in the reaction 1h, 2h, 3h, 4h are respectively adding 240uL is propionaldehyde. In the reaction process and reaction conditions for TLC, reaction conditions estimated raw material, when the raw material after the reaction is complete can stop the reaction (about 6h).2), stop heating after the reaction, cooling to room temperature, with 30 ml ethyl acetate extraction, the aqueous phase (the lower) the quality of the diluted for the fraction of 20% NaOH to adjust the pH to 8, in the process the alkali adjusting full stirring, not to allow the temperature of the solution is too high (i.e., not to exceed 60 °C). Then extracting with ethyl acetate three times, combined with the phase (the upper), pressure-reducing (pressure -0.08--0.10 MPa) concentrated to dry, yellow oily substance is about 1.532 g. Finally through the column chromatography the 2-ethyl -3, 6-dimethyl-pyrazine separated.As follows:The silica gel chromatography (built-in 200-300 purpose silica gel 30g), the resulting 1.532g for column chromatography yellow oily substance; for the mobile phase ethyl acetate: petroleum ether = 1:20 of the mixed solution, the total consumption of mobile phase about 500 ml; final 1.245g product---2-ethyl -3, 6-dimethyl-pyrazine (the purity is 95.6%). Yield 90.88%.
References:
Zhejiang University;Cheng, Jingli;Wang, Likun;Zhao, Yang;Zhao, Jinhao CN105237486, 2016, A Location in patent:Paragraph 0045; 0046; 0047; 0048; 0049; 0050
5625-46-7
99 suppliers
$32.50/5g

13360-65-1
77 suppliers
inquiry
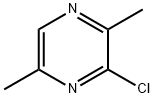
95-89-6
204 suppliers
$9.00/1g
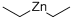
557-20-0
239 suppliers
$14.00/1g
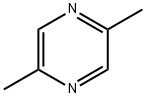
123-32-0
517 suppliers
$6.00/5g

13360-65-1
77 suppliers
inquiry