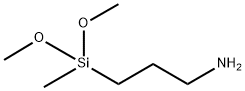
3-(Dimethoxymethylsilyl)propylamine synthesis
- Product Name:3-(Dimethoxymethylsilyl)propylamine
- CAS Number:3663-44-3
- Molecular formula:C6H17NO2Si
- Molecular Weight:163.29
16881-77-9
128 suppliers
$57.43/5g

107-11-9
0 suppliers
$29.09/25ml

3663-44-3
174 suppliers
$15.00/25g
Yield:3663-44-3 74%
Reaction Conditions:
in ethanol;
Steps:
3 Example 3
Example 3 Into the same reactor as used in Example 1, 17.1 parts of allylamine, 0.3 part of carbazole, 0.00005 part as platinum atom of the same platinum complex as in Example 1 were charged, 31.8 parts of methyldimethoxysilane were added dropwise at 40° C. over 5 minutes, followed further by continuing stirring for 4 hours while maintaining the liquid temperature at 60° C. After cooling, 1.7 parts of ethanol were added and distillation was carried out to give 36.2 parts of γ-aminopropylmethyldimethoxysilane boiling at 105° to 109° C./50 Torr. This corresponded to 74% yield based on the theoretical amount. The same reaction was carried out by use of 0.3 part of phenothiazine in place of carbazole. As a result, the yield of γ-aminopropylmethyldimethoxysilane was 35.2 parts, which corresponded to 72% yield based on the theoretical amount.
References:
US4649208,1987,A