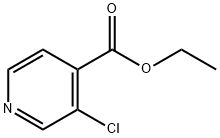
3-CHLOROISONICOTINIC ACID ETHYL ESTER synthesis
- Product Name:3-CHLOROISONICOTINIC ACID ETHYL ESTER
- CAS Number:211678-96-5
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61

64-17-5
756 suppliers
$10.00/50g

211678-96-5
62 suppliers
$20.00/1g
Yield:211678-96-5 94%
Reaction Conditions:
Stage #1: 3-chloro-isonicotinic acidwith thionyl chloride for 2.5 h;Heating / reflux;
Stage #2: ethanolwith N-ethyl-N,N-diisopropylamine at 0 - 20; for 18.17 h;
Steps:
7
3-Chloro-isonicotinic acid ethyl esterCl O( so N. y[00263] A suspension of 3-chloro-isonicotinic acid (1.0 g, 6.35 mmol) in thionyl chloride (10 ml) was heated under reflux for 2.5 hours. After cooling to ambient temperature, the solution was concentrated to dryness and then azeotroped with toluene (10 ml) to afford an oil. The resultant oil was added dropwise over 10 minutes to a cooled (O0C) solution of ethanol (15 ml) and DIPEA (5 ml). The reaction was stirred at room temperature for 18 hours then concentrated in vacuo before water (20 ml) was added. The solution was extracted with ethyl acetate (30 ml) and the organic phase was dried over sodium sulfate then concentrated to give the title compound as an orange oil (1.1 g, 94%). IH NMR (CDCl3, 400MHz) 8.72 (s, IH), 8.59 (d, J = 4.9 Hz, IH), 7.63 (dd, J = 4.9 Hz,
References:
WO2008/24724,2008,A1 Location in patent:Page/Page column 68-69

64-17-5
756 suppliers
$10.00/50g
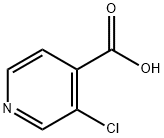
88912-27-0
192 suppliers
$14.00/1g

211678-96-5
62 suppliers
$20.00/1g

88912-27-0
192 suppliers
$14.00/1g

211678-96-5
62 suppliers
$20.00/1g