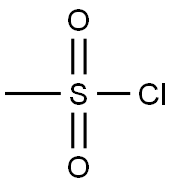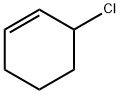
3-CHLOROCYCLOHEXENE synthesis
- Product Name:3-CHLOROCYCLOHEXENE
- CAS Number:2441-97-6
- Molecular formula:C6H9Cl
- Molecular Weight:116.59
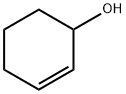
822-67-3
111 suppliers
$49.60/1g

2441-97-6
49 suppliers
$50.00/50mg
Yield: 81%
Reaction Conditions:
with 1-chloro-1-(dimethylamino)-2-methyl-1-propene in dichloromethane at 0 - 20; for 3 h;Inert atmosphere;
Steps:
4.2. General procedure for the reaction of a-haloenamines withallylic and propargylic alcohols
General procedure: Reactions were performed in an oven- or flame-dried three neckround-bottomed flask under dry argon and with magnetic stirring. The flask was equipped with a septum and a refrigerator isolated from moisture by an oil trap. A solution of alcohol in CDCl3 or CH2Cl2 was syringed in through the septum and cooled down at 0 °C. A solution of α-haloenamine (1.1 equiv.) was then syringed into the flask and the mixture was left at room temperature. The reaction was followed by 1H NMR. Yields were determined after removal of the solvent either by 1H NMR using an added standard (usually benzene or toluene) or by GLC. In some cases, the halides were purified by distillation or flash chromatography. The isolated yields were always very close to those measured by NMR or GLC. Most of the halogenation products obtained in this study were known compounds: their spectroscopic properties have been shown to be identical to those reported in the literature Therefore, no data or only 1H NMR are reported for these molecules.
References:
Ghosez, Léon;Munyemana, Fran?ois;Patiny, Luc [Tetrahedron,2021,vol. 89]
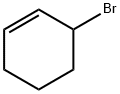
1521-51-3
174 suppliers
$14.00/1g

2441-97-6
49 suppliers
$50.00/50mg
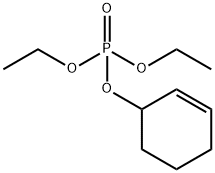
68735-62-6
1 suppliers
inquiry

2441-97-6
49 suppliers
$50.00/50mg
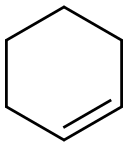
110-83-8
317 suppliers
$11.00/25g
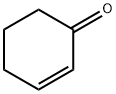
930-68-7
260 suppliers
$9.00/1g

2441-97-6
49 suppliers
$50.00/50mg

822-67-3
111 suppliers
$49.60/1g