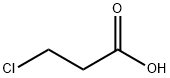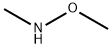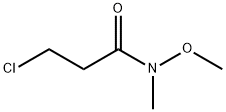
3-Chloro-N-methoxy-N-methyl-propionamide synthesis
- Product Name:3-Chloro-N-methoxy-N-methyl-propionamide
- CAS Number:1062512-53-1
- Molecular formula:C5H10ClNO2
- Molecular Weight:151.59
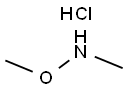
6638-79-5
572 suppliers
$6.00/25g
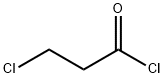
625-36-5
371 suppliers
$45.00/5g

1062512-53-1
11 suppliers
inquiry
Yield:1062512-53-1 99.9%
Reaction Conditions:
with potassium carbonate in diethyl ether;water at 0 - 20;Inert atmosphere;
Steps:
1 4.2. General procedure for the preparation of compounds 2-3
General procedure: To an aqueous solution of K2CO3 (2.0 equiv.) was added Et2O andN,O-dimethylhydroxyamine hydrochloride (1.5 equiv.). The resultingmixture was cooled at 0 °C and after 2 min the correspondingacyl chloride (1.0 equiv.) was added dropwise. The reaction wasstirred overnight until the room temperature was reached. Thereaction mixture was extracted with Et2O (2 5 mL) and washedwith water (5 mL) and brine (10 mL). The organic phase was dried(anhydrous Na2SO4), filtered and, after removal of the solvent underreduced pressure, the pure compounds 2-3 were obtained.4.2.1 3-Chloro-N-methoxy-N-methylpropanamide (2) By following the General Procedure, starting from 3-chloropropanoyl chloride (127?mg, 1.00?mmol, 1.0 equiv), K2CO3 (276?mg, 2.0?mmol, 2.0 equiv), N,O-dimethylhydroxyamine hydrochloride (146?mg, 1.5?mmol, 1.5 equiv), H2O (3?mL) and Et2O (3?mL), the desired product was obtained in a quantitative amount (>99.9%) (151?mg) as a bright yellow oil. 1H NMR (500?MHz, CDCl3) δ: 3.80 (t, J?=?6.9?Hz, 2H, CH2CH2Cl), 3.70 (s, 3H, NOCH3), 3.19 (s, 3H, NCH3), 2.91 (t, J?=?6.7?Hz, 2H, CH2CH2CO). 13C NMR (125?MHz, CDCl3) δ: 170.8, 61.4, 39.2, 35.0, 32.0.
References:
Rui, Marta;Rossino, Giacomo;Coniglio, Stefania;Monteleone, Stefania;Scuteri, Arianna;Malacrida, Alessio;Rossi, Daniela;Catenacci, Laura;Sorrenti, Milena;Paolillo, Mayra;Curti, Daniela;Venturini, Letizia;Schepmann, Dirk;Wünsch, Bernhard;Liedl, Klaus R.;Cavaletti, Guido;Pace, Vittorio;Urban, Ernst;Collina, Simona [European Journal of Medicinal Chemistry,2018,vol. 158,p. 353 - 370]