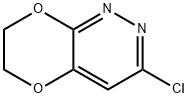
3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE synthesis
- Product Name:3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE
- CAS Number:943026-40-2
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
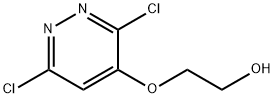
17284-80-9
1 suppliers
inquiry
![3-CHLORO-6,7-DIHYDRO[1,4]DIOXINO[2,3-C]PYRIDAZINE](/CAS/GIF/943026-40-2.gif)
943026-40-2
26 suppliers
inquiry
Yield:943026-40-2 77%
Reaction Conditions:
with lithium hydride in 1,4-dioxane at 20; for 1 h;
Steps:
10.A.c
(c) 3-Chloro-6,7-dihydro[1,4]dioxino[2,3-c]pyridazineA solution of 2-[(3,6-dichloro-4-pyridazinyl)oxy]ethanol containing some bromo-derivative (15.46 g; 0.0703 mol) in dry dioxan (1.2 L) was treated with lithium hydride (2.3 g; 0.28 mol) in portions and stirred at room temperature for 1 hour under argon, then heated at 110° C. overnight. The reaction mixture was quenched with wet dioxan, then iced-water. The solution was evaporated to half volume, taken to pH 8 with 5M hydrochloric acid and evaporated to dryness. Water was added and the residue was extracted 5× with chloroform, dried (sodium sulphate) and evaporated to afford a white solid (12.4 g, ca. 77%) (containing ca. 15% of a bromo species).MS (+ve ion electrospray) m/z 173/5 (Cl MH+); 217/9 (Br MH+)
References:
US2008/221110,2008,A1 Location in patent:Page/Page column 18