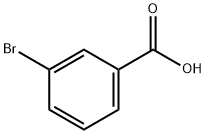
3-Bromobenzoic acid synthesis
- Product Name:3-Bromobenzoic acid
- CAS Number:585-76-2
- Molecular formula:C7H5BrO2
- Molecular Weight:201.02
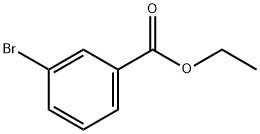
24398-88-7
162 suppliers
$5.00/1g

585-76-2
362 suppliers
$6.00/5g
Yield:585-76-2 99 %Chromat.
Reaction Conditions:
with water monomer;lithium hydroxide monohydrate in tetrahydrofuran at 25; for 6 h;Reagent/catalyst;
Steps:
General Procedure for Hydrolysis of Esters
General procedure: To a THF (1.0 mL) solution of ester (1.0 mmol) was added a 3.0 mol/L solution of hydroxide (LiOH, NaOH, or KOH) (1.0 mL, 3.0 mmol), and the reaction mixture was stirred at 25 ± 0.02 °C for 0.25-60 h (see Tables 1-4). The reaction was then quenched with 1.0 mol/L HCl (9.0 mL) for 3 min, and the mixture was adjusted accurately to a 250-mL volume with aqueous CH3CN (50% (v/v)). The resulting diluted solution was used for the HPLC an sis as a sample solution with TSkgel ODS-80Ts (150 × 4.6 mm) as a separation column. In the mobile phase, CH3CN/phosphate buffer (1/15 mol/L,pH 7.0) = 1 : 1 (v/v) or CH3CN/H2O (0.1% trifluoroacetic acid(TFA) (v/v)) = 1 : 1 (v/v); flow rate, 1.0 mL/min; measurement at room temperature (20-25 °C); detection wavelength,254 nm. The yield (%) was determined by utilizing the peak area ratios of the reaction mixture to that of the standard acid in the chromatogram.
References:
Hayashi, Kazuhiko;Ichimaru, Yoshimi;Sugiura, Kirara;Maeda, Azusa;Harada, Yumi;Kojima, Yuki;Nakayama, Kanae;Imai, Masanori [Chemical and Pharmaceutical Bulletin,2021,vol. 69,# 6,p. 581 - 594]
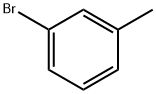
591-17-3
258 suppliers
$10.00/10g

585-76-2
362 suppliers
$6.00/5g
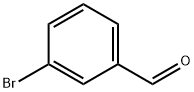
3132-99-8
502 suppliers
$5.00/10g

585-76-2
362 suppliers
$6.00/5g
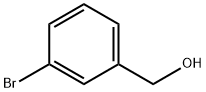
15852-73-0
282 suppliers
$11.00/25g

585-76-2
362 suppliers
$6.00/5g
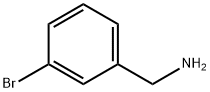
10269-01-9
200 suppliers
$8.00/1g

585-76-2
362 suppliers
$6.00/5g