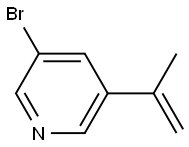
3-BroMo-5-(prop-1-en-2-yl)pyridine synthesis
- Product Name:3-BroMo-5-(prop-1-en-2-yl)pyridine
- CAS Number:40472-88-6
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08
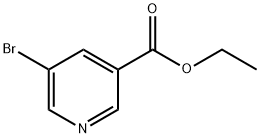
20986-40-7
271 suppliers
$6.00/10g
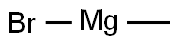
75-16-1
270 suppliers
$12.00/10ml

40472-88-6
60 suppliers
$45.00/10mg
Yield: 92%
Reaction Conditions:
Stage #1:ethyl 5-bromo-3-pyridinecarboxylate;methylmagnesium bromide in tetrahydrofuran;diethyl ether at 30;
Stage #2: with water;ammonium chloride in tetrahydrofuran;diethyl etherCooling with ice;
Steps:
3.1
Add a solution of ethyl 5-bromopyridine-3-carboxylate (22.5 g, 98 mmol) in tetrahydrofuran (350 m L) to a solution of 3M methylmagnesium bromide in diethyl ether (98 mL, 293 mmol, 3 eq.) with internal temperature below 30'C. Upon completion of reaction, cool the mixture in an ice bath and quench with saturated aqueous ammonium chloride and keep stirring until most solids dissolve. Add water (500 mL) and extract with ethyl acetate (3×500 mL). Dry the organic layer over sodium sulfate, filter and concentrate the organic layer to a residue. Purify the residue by silica gel column chromatography eluting with solvent of ethyl acetate:hexane (1:1), to afford the titled product as an oil (19.4 g, 92%). MS (ESI) m/z (M+H)+214.9, 216.9.
References:
ELI LILLY AND COMPANY US2012/184577, 2012, A1 Location in patent:Page/Page column 3
29681-44-5
298 suppliers
$6.00/5g
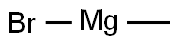
75-16-1
270 suppliers
$12.00/10ml

40472-88-6
60 suppliers
$45.00/10mg
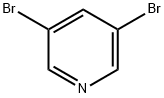
625-92-3
490 suppliers
$6.00/10g
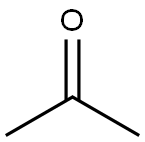
67-64-1
6 suppliers
$17.30/10ml

40472-88-6
60 suppliers
$45.00/10mg
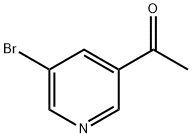
38940-62-4
233 suppliers
$6.00/250mg
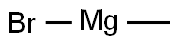
75-16-1
270 suppliers
$12.00/10ml

40472-88-6
60 suppliers
$45.00/10mg
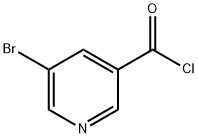
39620-02-5
71 suppliers
$82.00/10g
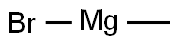
75-16-1
270 suppliers
$12.00/10ml

40472-88-6
60 suppliers
$45.00/10mg