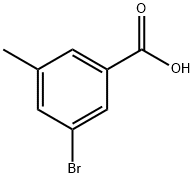
3-bromo-5-methylbenzoic acid synthesis
- Product Name:3-bromo-5-methylbenzoic acid
- CAS Number:58530-13-5
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
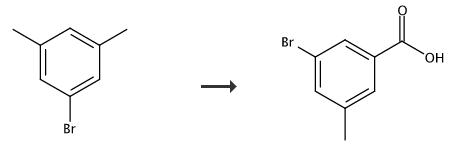
648439-19-4
49 suppliers
$45.00/100mg

58530-13-5
124 suppliers
$8.00/1g
Yield: 60%
Reaction Conditions:
with potassium permanganate in water;acetone for 1 h;Reflux;
Steps:
43 3-Bromo-5-methylbenzoic acid
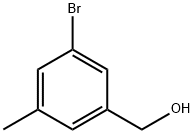
Example 43 3-Bromo-5-methylbenzoic acid A solution of KMnO4 (39.3 g, 249 mmol) in water (600 mL) was added slowly to a solution of (3-bromo-5-methylphenyl)methanol (25.0 g, 124 mmol) in acetone (500 mL). The mixture was kept at reflux for 60 mins. After cooling to room temperature, the mixture was acidified with HCl (2N, 100 mL). A brown precipitate formed and was dissolved by adding a solution of saturated sodium bicarbonate (100 mL); then acetone was evaporated in vacuum. Ammonia (150 mL) was added. The mixture was filtered over Celite and the filtrate acidified with concentrated HCl. The product was extracted with diethyl ether (3*150 mL). The combined organic phases were dried (Na2SO4) and concentrated in vacuum to obtain 16.0 g of the acid, 3-bromo-5-methylbenzoic acid, as white crystals (yield: 60%). 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H), 7.85-7.84 (m, 1H), 7.58 (s, 1H), 2.40 (s, 3H).
References:
Glaxo Group Limited US2009/203657, 2009, A1 Location in patent:Page/Page column 53
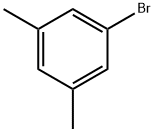
556-96-7
381 suppliers
$6.00/5g

58530-13-5
124 suppliers
$8.00/1g
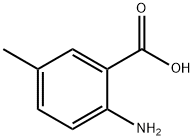
2941-78-8
487 suppliers
$5.00/10g

58530-13-5
124 suppliers
$8.00/1g
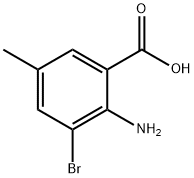
13091-43-5
137 suppliers
$9.00/250mg

58530-13-5
124 suppliers
$8.00/1g
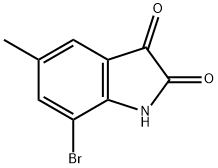
108938-16-5
52 suppliers
$52.00/100 mg

58530-13-5
124 suppliers
$8.00/1g