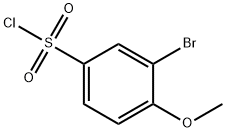
3-BROMO-4-METHOXY-BENZENESULFONYL CHLORIDE synthesis
- Product Name:3-BROMO-4-METHOXY-BENZENESULFONYL CHLORIDE
- CAS Number:23094-96-4
- Molecular formula:C7H6BrClO3S
- Molecular Weight:285.54
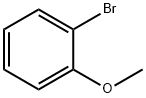
578-57-4
305 suppliers
$6.00/10g

23094-96-4
60 suppliers
$10.00/100mg
Yield: 98%
Reaction Conditions:
with chlorosulfonic acid in dichloromethane at -5 - 20; for 1.5 h;
Steps:
6 Reference 6; 3-BROMO-N-TERT-BUTYL-4-METHOXYBENZENESULFONAMIDE
L-BROMO-2-METHOXY-BENZENE (1.87 g, 10.0 mmol) was dissolved in chloroform (5 mL) and the solution was cooled in an ice-salt bath to-5° C to 0° C. The cooled solution was carefully charged with CHLOROSULFONIC acid (2.0 mL, 30.0 mmol) over 30 minutes and the mixture was allowed to warm to room temperature over 1 hour. The mixture then was poured onto chopped ice and transferred to a separatory funnel. The aqueous layer was separated and extracted (X2). The combined organic layers were dried and concentrated to give 3-bromo-4-methoxyphenylsulfonyl chloride (2.80 g, 98%). 3-Bromo-4-methoxyphenylsulfonyl chloride (2.80 g, 9.8 mmol) was dissolved in dichloromethane (30 mL) and the solution was treated with triethylamine (1.76 mL, 12.6 mmol), followed by the dropwise addition of t-butylamine (1.33 mL, 12.6 mmol). The mixture was allowed to stand for 2 hours and then was poured onto a mixture of 5% citric acid solution and dichloromethane. The organic layer was separated and the aqueous layer was extracted with dichloromethane/ (X2). The combined organic layers were dried and concentrated. Crystallization of the crude solid from ethyl acetate/hexane gave 3-BROMO-N- tert-butyl-4-methoxybenzenesulfonamide (2.43 g, 77%)
References:
AXYS PHARMACEUTICALS, INC. WO2004/50637, 2004, A2 Location in patent:Page 68-69