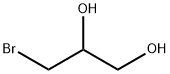
3-BROMO-1,2-PROPANEDIOL synthesis
- Product Name:3-BROMO-1,2-PROPANEDIOL
- CAS Number:4704-77-2
- Molecular formula:C3H7BrO2
- Molecular Weight:154.99
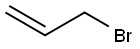
106-95-6
433 suppliers
$10.00/5g
75-65-0
770 suppliers
$10.00/10ml

4704-77-2
75 suppliers
$29.00/100mg
Yield:-
Reaction Conditions:
with sodium sulfite in water;ethyl acetate
Steps:
21 Example 21
Example 21 Chiral synthesis ofnitrate IVd A 50 mL round-bottom flask, equipped with a magnetic stirrer, was charged with 10 mL of tert-butyl alcohol, 10 mL of water, and 2.8 g of a catalyst (AD-mix-β, Aldrich: K.B.Sharpless, W.Amberg, Y.L.Bennani, G.A.Crispino, J.Hartung, K.-S.Jeong, H.-L.Kwong, K.Morikawa, Z.-M.Wang, D.Xu, X.-L.Zhang, J.Org.Chem. (1992) 57, 2768-2771). Stirring at room temperature produced two clear phases; the lower aqueous phase appears bright yellow. The mixture was cooled to 4° and 0.2 mL (2 mmol) of allylbromide was added at once, and the heterogeneous slurry was stirred vigorously at 4-5° for 2.5 h (monitoring by TLC hexane:methanol=1:9). While the mixture was stirred at 0°C, solid sodium sulfite.(3 g) was added and the mixture was allowed to warm to room temperature and stirred for 1 h. Then 20 mL of ethyl acetate was added to the reaction mixture, and after separation of the layers, the aqueous phase was further extracted with ethyl acetate. The combined organic extracts were dried (MgSO4), concentrated in vacuo and purified by flash chromatography on silica (hexane:methanol=1:9, Rf 0.5), to yield chiral 1-bromo-2,3-propanediol. Yield 0.2 g (55.5%).
References:
QUEEN'S UNIVERSITY AT KINGSTON EP1246625, 2004, B1

3132-64-7
132 suppliers
$20.00/1 G

4704-77-2
75 suppliers
$29.00/100mg
556-52-5
285 suppliers
$17.67/1gm:

4704-77-2
75 suppliers
$29.00/100mg

107-18-6
0 suppliers
$24.60/100ml

4704-77-2
75 suppliers
$29.00/100mg
56-81-5
1700 suppliers
$5.00/25g

4704-77-2
75 suppliers
$29.00/100mg