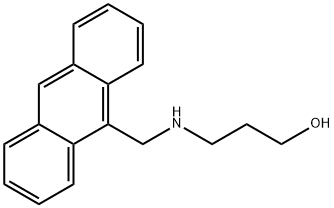
3-[(ANTHRACEN-9-YLMETHYL)-AMINO]-PROPAN-1-OL synthesis
- Product Name:3-[(ANTHRACEN-9-YLMETHYL)-AMINO]-PROPAN-1-OL
- CAS Number:14131-13-6
- Molecular formula:C18H19NO
- Molecular Weight:265.35
Yield:14131-13-6 78%
Reaction Conditions:
Stage #1: 9-anthracene aldehyde;propan-1-ol-3-amine in methanol;dichloromethane at 20;
Stage #2: with sodium tetrahydroborate in methanol;dichloromethane at 0 - 20;
Steps:
14 Synthesis of 3-[(Anthracen-9-ylmethyl)-amino]-propan-1-ol, 14a
To a stirred solution of 3-amino -1-propanol, 15a (0.87 g, 11.65 mmol) in 25% MeOH/CH2Cl2 (20 mL), was added a solution of aldehyde 8 (2.00 g, 9.7 mmol) in 25% MeOH/CH2Cl2 (15 mL) under N2. The mixture was stirred at room temperature overnight until the imine formation was complete (monitored by NMR). The solvent was removed in vacuo, the solid residue dissolved in 50% MeOH/CH2Cl2 (40 mL) and the solution was cooled to 0° C. NaBH4 (29.1 mmol) was added in small portions to the solution and the mixture was stirred at rt overnight. The solvent was removed in vacuo, the solid residue dissolved in CH2Cl2 (40 mL) and washed with 10% aq. Na2CO3 solution (3×30 mL). The CH2Cl2 layer was separated, dried over anhydrous Na2SO4, filtered and removed in vacuo to give an oily residue. The oil was purified by flash column chromatography (6% MeOH/CHCl3) to yield the product 14a as a pale yellow thick oil (78%), Rf=0.3 (6% MeOH/CHCl3); 1H NMR (CDCl3) δ 8.39 (s, 1H), 8.27 (d, 2H), 7.99 (d, 2H), 7.52 (m, 2H), 7.45 (m, 2H), 4.71 (s, 2H), 3.79 (t, 2H), 3.09 (t, 2H), 1.74 (q, 2H).
References:
US2007/213397,2007,A1 Location in patent:Page/Page column 7
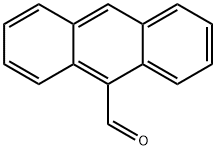
642-31-9
345 suppliers
$10.00/5g
![3-[(ANTHRACEN-9-YLMETHYL)-AMINO]-PROPAN-1-OL](/CAS/GIF/14131-13-6.gif)
14131-13-6
7 suppliers
inquiry
