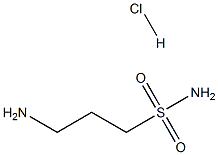
3-AMinopropane-1-sulfonaMide, HCl synthesis
- Product Name:3-AMinopropane-1-sulfonaMide, HCl
- CAS Number:104458-33-5
- Molecular formula:C3H11ClN2O2S
- Molecular Weight:174.6496
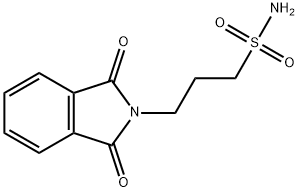
6712-89-6
4 suppliers
inquiry

104458-33-5
15 suppliers
$45.00/25mg
Yield:104458-33-5 74%
Reaction Conditions:
Stage #1: 3‐(1,3‐dioxo‐2,3‐dihydro‐1H‐isoindol‐2‐yl)propane‐1‐sulfonamidewith hydrazine in ethanol;water; for 4 h;Heating / reflux;
Stage #2: with hydrogenchloride in water; pH=4;
Steps:
A mixture of 3-amino-1-propanesulphonic acid (9.96g, 71.55mmol), potassium acetate (7.01 g, 71.55mmol) and glacial acetic acid (30ml) was heated to reflux, with stirring for 10 mins. Phthalic anhydride (10.6g, 71.55mmol) was added to the suspension and the mixture refluxed for a further 24 hrs. The reaction was cooled and left to stand for a further 2 days. No precipitation occurred so ether (400ml) was added, resulting in a white solid. This was filtered off under vacuum and washed well with ether (200ml) to afford potassium 3-(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1-propanesulfonate as a white solid (22.9g, 100%). Potassium 3-(1,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1-propanesulfonate (20g,65mmol) was heated with stirring under reflux with toluene (100ml) using Dean-Stark apparatus for 24 hrs. Phosphorous pentachloride (9.8g, 47.2mmol) was added portion wise to the suspension and heated at reflux for 1 hour, resulting in a green suspension. A further quantity of phosphorous pentachloride (10.5g, 50.42mmol) was added portion wise under nitrogen and the resulting solution refluxed for a further 5 hrs. The solution was left to stand overnight, distilled at atmospheric pressure and evaporated to give an oil. Crushed ice (100ml) was added giving a sticky solid which hardened and dried on filtering off under vacuum. The filtrate was decanted off, redissolved in toluene and evaporated again to give an oil. A further quantity of crushed ice (100ml) was added, the solid filtered off and washed with water. The 2 batches of solid were combined and dried (6O0C) to give 3-(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1-propanesulfonyl chloride as a beige solid (14.77g, 79%). 3-(1 ,3-Dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1-propanesulfonyl chloride (5.Og, 17.4mmol) was added in portions, with stirring to liquid ammonia. Stirring at -280C was continued for 30 mins. The cooling bath was removed, the reaction mixture warmed to room temperature and the ammonia allowed to boil off. The residue was treated with acetic acid (glacial, 4ml) in water (10ml). The resulting brown solution was evaporated to dryness giving viscous orange/brown oil (4g). This failed to solidify on treating with ether so it was applied in the minimum volume of methanol to a flash column of silica (Merck 9385, 230ml, 4.2cm wide). Elution with DCM-methanol-0.88 ammonia (890:100:10) afforded on evaporation 3-(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1-propanesulfonamide as a cream foam (1.52g, 33%). A solution of 3-(1 ,3-dioxo-1 ,3-dihydro-2H-isoindol-2-yl)-1- propanesulfonamide (1.52g, 5.7mmol) in ethanol (21ml) and water (0.6ml) was treated with hydrazine hydrate (0.2ml, 0.2g, 6.23mmol) and heated under reflux under nitrogen for
References:
WO2008/34860,2008,A1 Location in patent:Page/Page column 115-116
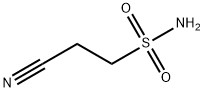
97716-80-8
6 suppliers
inquiry

104458-33-5
15 suppliers
$45.00/25mg
