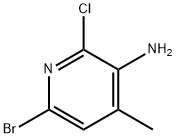
3-AMINO-6-BROMO-2-CHLORO-4-METHYLPYRIDINE synthesis
- Product Name:3-AMINO-6-BROMO-2-CHLORO-4-METHYLPYRIDINE
- CAS Number:1038920-08-9
- Molecular formula:C6H6BrClN2
- Molecular Weight:221.48
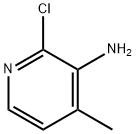
133627-45-9
492 suppliers
$9.00/1g

1038920-08-9
30 suppliers
inquiry
Yield:1038920-08-9 94%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione in dichloromethane at 0 - 20; for 1.66667 h;Product distribution / selectivity;
Steps:
10.b.1; 10.c.1
Example 10b. Alternative synthetic route to (S)-1-(3-((1 S)-5-(2-(1 H-tetrazol-5- yl)phenyl)-2l3-dihydro-1H-inden-1-yl)-2-ethyl-7-methyl-3H-imida20[4,5-b]pyridin-5-yl)-2- methylpropan-1-ol; Step 1. 6-Bromo-2-chloro-4-methyl-pyridin-3-ylamine [6-bromo-2-chloro-4-methylpyridin-3-amine]; 3-Amino-2-chloro-4-methylpyridine (50.0 g, 351 mmol) was dissolved in dichloromethane (500 mL) under nitrogen and the solution cooled in an ice bath to between 0-1 0C. Dibromo-5,5-dimethylhydantoin (51.1 g, 179 mmol) was added in 5 portions over 40 min. After the addition was finished, the mixture was stirred at room temperature for 2 hours. The reaction mixture was passed through a short pad of silica gel, eluted with 30% ether/ dichloromethane (1 L). The mixture was concentrated and heptane was added. The precipitate was collected by filtration, washed with heptane, and air dried to give 6-bromo-2-chloro-4-methyl-pyridin-3-ylamine (73.3 g, 94%). MS: 223.0 (APCI)+; 220.9 (APCI)"; Example 10c. (S)-1-(3-((1S)-5-(2-(1H-tetrazol-5-yl)phenyl)-2,3-dihydro-1H-inden-1-yl)-2- ethyl-7-methyl-3H-imidazo[4,5-b]pyridin-5-yl)-2-methylpropan-1-ol; Step 1 Bromo-2-chloro-4-methyl-pyridin-3-ylamine; 3-Amino-2-chloro-4-methy.pyridine (50.0 g, 351 mmol) was dissolved in DCM (500 mL) under nitrogen and cooled the solution in an ice bath to between 0-1 0C.
References:
WO2008/84303,2008,A1 Location in patent:Page/Page column 76; 80-81