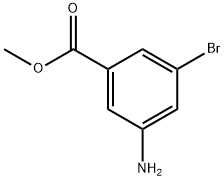
Methyl 3-amino-5-bromobenzoate synthesis
- Product Name:Methyl 3-amino-5-bromobenzoate
- CAS Number:706791-83-5
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
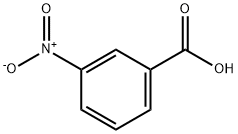
121-92-6
573 suppliers
$6.00/25g

706791-83-5
112 suppliers
$8.00/250mg
Yield:-
Steps:
Multi-step reaction with 3 steps
1: sulfuric acid; N-Bromosuccinimide / 2 h / 60 °C
2: sodium carbonate / N,N-dimethyl-formamide / 8 h / 60 °C
3: ammonium chloride; iron / ethanol; water / 1 h / 80 °C
References:
Kuntz, Kevin W.;Campbell, John E.;Keilhack, Heike;Pollock, Roy M.;Knutson, Sarah K.;Porter-Scott, Margaret;Richon, Victoria M.;Sneeringer, Chris J.;Wigle, Tim J.;Allain, Christina J.;Majer, Christina R.;Moyer, Mikel P.;Copeland, Robert A.;Chesworth, Richard [Journal of Medicinal Chemistry,2016,vol. 59,# 4,p. 1556 - 1564]
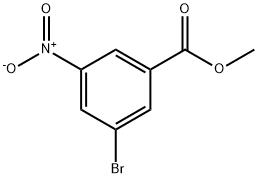
6307-87-5
116 suppliers
$9.00/1g

706791-83-5
112 suppliers
$8.00/250mg

67-56-1
757 suppliers
$7.29/5ml-f
42237-85-4
134 suppliers
$8.00/1g

706791-83-5
112 suppliers
$8.00/250mg
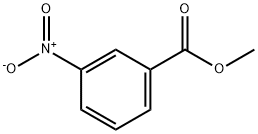
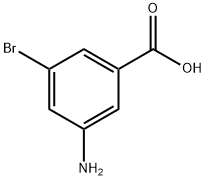
618-95-1
205 suppliers
$14.00/5g

706791-83-5
112 suppliers
$8.00/250mg
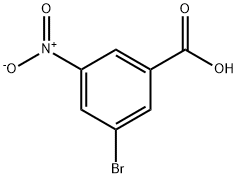
6307-83-1
214 suppliers
$5.00/250mg

706791-83-5
112 suppliers
$8.00/250mg