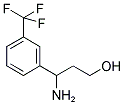
3-AMINO-3-(3-TRIFLUOROMETHYL-PHENYL)-PROPAN-1-OL synthesis
- Product Name:3-AMINO-3-(3-TRIFLUOROMETHYL-PHENYL)-PROPAN-1-OL
- CAS Number:683221-00-3
- Molecular formula:C10H12F3NO
- Molecular Weight:219.2
![3-AMINO-3-[3-(TRIFLUOROMETHYL)PHENYL]PROPANOIC ACID](/CAS/GIF/143438-91-9.gif)
143438-91-9
77 suppliers
$21.00/250mg

683221-00-3
18 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3-amino-3-(3-(trifluoromethyl)phenyl)propanoic acidwith borane-THF in tetrahydrofuran;Inert atmosphere;Cooling with ice bath;Reflux;
Stage #2: with water in tetrahydrofuran;
Stage #3: with sodium hydroxide in tetrahydrofuran;water;
Steps:
127A
Example 127A 3-Amino-3-[3-(trifluoromethyl)phenyl]propan-1-ol With ice cooling and under argon, 10.9 ml (10.94 mmol) of borane-tetrahydrofuran complex (1M in THF) were introduced. Then 850 mg (3.65 mmol) of 3-amino-3-[3-(trifluoromethyl)phenyl]propanoic acid were added. After 5 minutes, the cooling bath was removed and the mixture was stirred at RT overnight and for 4 h at reflux. After cooling to RT, pieces of ice were added until the evolution of gas came to an end. The mixture was rendered alkaline with 1M aqueous sodium hydroxide solution, diluted with water to a volume of approximately 150 ml, and extracted three times with dichloromethane. The combined organic phases were dried over sodium sulphate, filtered and freed from the solvent on a rotary evaporator. Drying in an HV gave 744 mg (85% of theory) of the title compound in 92% purity. LC/MS [Method 4]: Rt=0.45 min; m/z=220 (M+H)+. 1H-NMR (400 MHz, DMSO-d6): δ=7.71 (s, 1H), 7.60-7.67 (m, 1H), 7.49-7.58 (m, 2H), 4.55 (br.s, 1H), 3.97-4.04 (dd, 1H), 3.34-3.50 (m, 2H), 2.00 (br. s., 2H), 1.59-1.78 (m, 2H).
References:
US2010/261771,2010,A1 Location in patent:Page/Page column 52