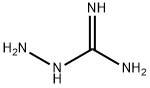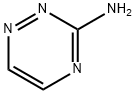
3-AMINO-1,2,4-TRIAZINE synthesis
- Product Name:3-AMINO-1,2,4-TRIAZINE
- CAS Number:1120-99-6
- Molecular formula:C3H4N4
- Molecular Weight:96.09
Yield:1120-99-6 90%
Reaction Conditions:
in water at 20; for 18 h;
Steps:
22 1,2,4-Triazin-3-amine (16)
Example 22 1,2,4-Triazin-3-amine (16) An aqueous solution of glyoxal (57 Kg of 40 wt % aqueous solution, 393 mol, 0.73 equiv) was added to a suspension of aminoguanidine bicarbonate (73 Kg, 536.3 mol) in water (400 L) at room temperature. The evolution of carbon dioxide (CO2) began almost immediately. The reaction mixture was then stirred at room temperature for 18 h and the evolution of gas had virtually ceased after about 2 h. The reaction mixture was then filtered, and the filtrate was evaporated to dryness under the reduced pressure. The residue was then extracted with cold methanol (MeOH, 3*120 L), and the combined methanol solution was cooled down to 0-5° C. before being filtered to remove the residual solids. The filtrate was then concentrated under the reduced pressure, and the residue was recrystallized in acetonitrile to afford 1,2,4-triazin-3-amine (16, 34 Kg, 37.76 Kg theoretical, 90% yield) as fine, white needles.
References:
US2009/291956,2009,A1 Location in patent:Page/Page column 25-26

2582-30-1
380 suppliers
$18.00/25g
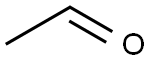
75-07-0
422 suppliers
$14.00/5mL

1120-99-6
171 suppliers
$8.00/1g