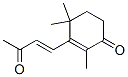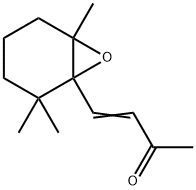
4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one synthesis
- Product Name:4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one
- CAS Number:23267-57-4
- Molecular formula:C13H20O2
- Molecular Weight:208.3
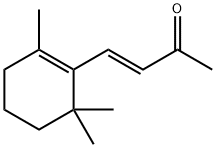
79-77-6
249 suppliers
$10.00/25g
![4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one](/CAS/GIF/23267-57-4.gif)
23267-57-4
24 suppliers
inquiry
Yield:23267-57-4 68%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 20; for 12 h;Cooling with ice;
Steps:
13 4.3.13
4-(1',2'-Epoxy-2',6',6'-trimethylcyclohexyl)-3-buten-2-one (12)
m-Chloroperoxybenzoic acid (13.4 mg, 0.078 mmol, 1.5 equiv) was added to a solution of (+)-β-ionone (11 μl, 0.052 mmol, 1 equiv) in 600 μl of methylene chloride, being cooled in an ice bath.
The reaction mixture was stirred at room temperature for about 12 h and then filtered and washed with aqueous ammonium hydrogen carbonate 0.1 M.
The organic phase was dried with anhydrous sodium sulfate, and evaporated under reduced pressure.
The residue was purified by RP-HPLC to obtain the title compound (7.4 mg, yield of 68%) as an yellow oil.
ESI-MS m/z: 208.2.
1H NMR (DMSO-d6, 300 MHz): T = 25 °C; 0.94 [s, 3H, 8']; 1.14[s, 6H, 7'e 9']; 1.40 [m, 1H, 5']; 1.30-1.9 [m, 6H, 4', 3', 5']; 2.30 [s, 3H, CH3CO]; 6.10 [d, 1H, 3, J = 16 Hz]; 7.01 [dd, 1H, 4, J = 16 Hz].
References:
Bellotti, Marta;Salis, Annalisa;Grozio, Alessia;Damonte, Gianluca;Vigliarolo, Tiziana;Galatini, Andrea;Zocchi, Elena;Benatti, Umberto;Millo, Enrico [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 1,p. 22 - 32]

79-77-6
249 suppliers
$10.00/25g
![4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-3-buten-2-one](/CAS/GIF/23267-57-4.gif)
23267-57-4
24 suppliers
inquiry