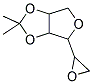
2,2-DIMETHYL-4-(2-OXIRANYL)TETRAHYDROFURO[3,4-D][1,3]DIOXOLE synthesis
- Product Name:2,2-DIMETHYL-4-(2-OXIRANYL)TETRAHYDROFURO[3,4-D][1,3]DIOXOLE
- CAS Number:226709-43-9
- Molecular formula:C9H14O4
- Molecular Weight:186.21
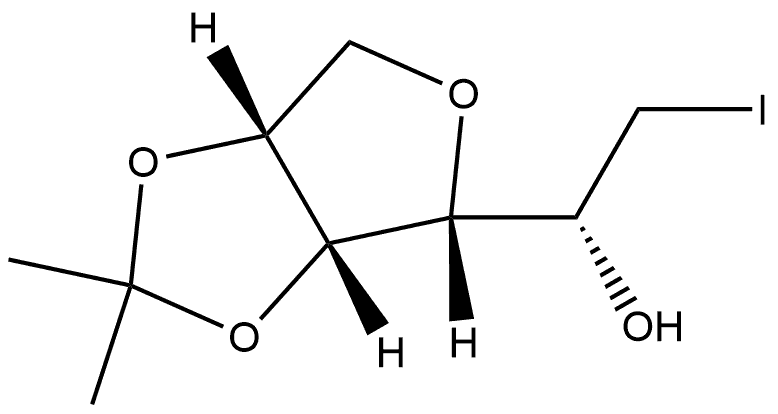
188778-70-3
0 suppliers
inquiry
![2,2-DIMETHYL-4-(2-OXIRANYL)TETRAHYDROFURO[3,4-D][1,3]DIOXOLE](/StructureFile/ChemBookStructure6/GIF/CB0740742.gif)
226709-43-9
11 suppliers
$362.00/500mg
Yield:226709-43-9 90%
Reaction Conditions:
with sodium hydride in 1,4-dioxane;mineral oil at 50;Cooling with ice;
Steps:
3.3. General procedure for the synthesis of dianhydrosugars (12 and 13)
To a solution of the iodoalcohol (10/11, 1 g) in anhydrous dioxane (10 mL) maintained over an ice-bath was added NaH (60% suspension in mineral oil, 1.1 mol equiv) and stirred. After 15 min, continuation of the reaction for a further period of 12 h was carried out at 50 °C. After the completion of the reaction it was filtered through a Celite-bed and the filtrate was concentrated under reduced pressure. The crude product was purified by column chromatography.
References:
Mugunthan;Sriram, Dharmarajan;Yogeeswari, Perumal;Kartha, K. P. Ravindranathan [Carbohydrate Research,2011,vol. 346,# 13,p. 1760 - 1766] Location in patent:experimental part
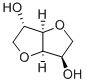
652-67-5
406 suppliers
$5.00/25g
![2,2-DIMETHYL-4-(2-OXIRANYL)TETRAHYDROFURO[3,4-D][1,3]DIOXOLE](/StructureFile/ChemBookStructure6/GIF/CB0740742.gif)
226709-43-9
11 suppliers
$362.00/500mg