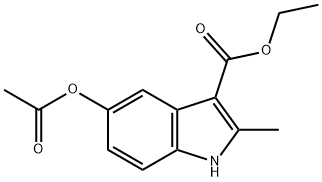
1H-Indole-3-carboxylic acid, 5-(acetyloxy)-2-Methyl-, ethyl ester synthesis
- Product Name:1H-Indole-3-carboxylic acid, 5-(acetyloxy)-2-Methyl-, ethyl ester
- CAS Number:20862-91-3
- Molecular formula:C14H15NO4
- Molecular Weight:261.27
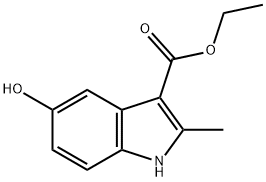
7598-91-6
100 suppliers
$60.00/50mg
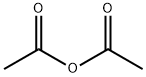
108-24-7
5 suppliers
$14.00/250ML

20862-91-3
2 suppliers
inquiry
Yield:20862-91-3 96%
Reaction Conditions:
with pyridine for 1 h;Inert atmosphere;Reflux;
Steps:
Ethyl' 5-aceioxy-2-methyM tf-indo 3 earboxylate 1a: References:
WO2018/112128,2018,A1 Location in patent:Page/Page column 9-11
Acetic anhydride (25,9 ml, 274 mmc- 20 eq.) was added to a stirred solution of ethyl 5-hydroxy-2-methy|-1 W-lndole-3-carbo ylate 1 (3.00 g, 13.6 mmoi, 1 ,0 eq.) in pyridine (3,32 mL, 41 .1 mmoi, 3.0 eq,) and the reaction heated to reflux. After 1 h, the reaction was allowed to cool back to rt before pouring- the mixture into a solution of aqueous saturated sodium bicarbonate (40 mL). The product .was. extracted with ethyl acetate (3 x 40 ml) and the combined organic layers, were washed with wate (40 mL), to yield the product as a white solid which was used without. further purification (3.4 g, 96%). NM : δ (40Q MHz, CDCls) 8.34 (1 H, s; H), 7.75 (1H, s, }, 7.21 (1 H , d, J 8.5, He), 6.89 (1 H, d, J 8.5, 7 4.38 (2H, q, J 7.1 , C02CH2CH3), 2.71 (3H, e, CiCH3), 2.34 (3H, s, CqaCH3), 1.43 (3H, t, J 7.1 , CO20H2C3). c (100 MHz, CQCb) 170.8 (CG2CH3}, 165.8 (COaEt), 1.45.9 (Gs), 145.3 (G2), 132.4 (C8). 127,9 {C3), 116.3 (C6), 113.8 (C<>, 111.2
100-02-7
19 suppliers
$11.00/5G

20862-91-3
2 suppliers
inquiry
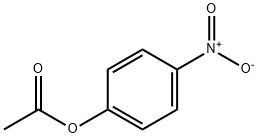
830-03-5
189 suppliers
$10.00/5g

20862-91-3
2 suppliers
inquiry
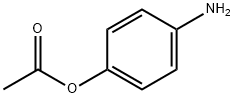
13871-68-6
83 suppliers
inquiry

20862-91-3
2 suppliers
inquiry