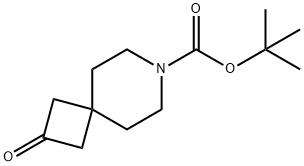
tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate synthesis
- Product Name:tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate
- CAS Number:203661-69-2
- Molecular formula:C13H21NO3
- Molecular Weight:239.31
![tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/20180601/GIF/396653-31-9.gif)
396653-31-9
14 suppliers
inquiry
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
169 suppliers
$8.00/250mg
Yield:203661-69-2 80%
Reaction Conditions:
with ammonium chloride;zinc in methanol at 0 - 30;Product distribution / selectivity;
Steps:
A
Synthesis of tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate; Method A. A mixture of ammonium chloride (832 g, 15 mol) and methanol (11 L) in a 20 L reactor was stirred and cooled to 0° C. A solution of tert-butyl 1,1-dichloro-2-oxo-7-azaspiro[3.5]nonane-7-carboxylate (1393 g, 4.5 mol) in methanol (2.5 L) was added to the mixture, followed by a 500 mL methanol wash. The mixture was cooled to 0° C. and treated with zinc dust (1400 g) in 50 g portions, keeping the reaction temperature below 8° C. with 0° C. cooling. After the first 250 g of zinc was added, the jacket temperature was raised to 12° C., and the next 500 g of zinc was added in 100 g portions over two hours. The reaction temperature was raised to 15° C. and the remaining 650 g of zinc was added in 100 g portions over 1 h. The temperature was raised to 25° C. and treated with an additional 472 g of zinc. The reaction was stirred at 30° C. for 1 h. The mixture was filtered through a pad of celite, washing with methanol (2 L). The filtrate was concentrated to 1.2 L and diluted with MTBE (3 L). The organic was extracted with saturated ammonium chloride solution (2×1 L) and brine (1 L). The organic layer was filtered through magnesol (1 kg), washing with MTBE (2 L). The filtrate was concentrated to give a yellow oil (870 g) which was dissolved in hexane (2 L), cooled to 0° C., and filtered, washing with cold hexane (1 L) to give the title compound (740 g). The filtrate was concentrated to give an oil (130 g) which was combined with additional product (83 g) from re-extractions from the aqueous phases with MTBE (2 L) which were passed through the same magnesol cake with MTBE (2 L). The combined 213 g of oil was purified by short path distillation using a wiped film evaporator at 130° C. and 500 mtorr to yield 145 g which was crystallized from hexane to give 120 g of the title compound. Combination of the 740 g and 120 g batches gave the title compound as a white solid (860 g, 80%). 1H NMR (400 MHz, CDCl3) δ ppm 3.40-3.37 (4H, t, J=5.44 Hz), 2.8 (4H, s), 1.69-1.67 (4H, t, J=5.36 Hz), 1.45 (9H, s); GCMS m/z 239.
References:
Pfizer Inc. US2010/113465, 2010, A1 Location in patent:Page/Page column 15-16
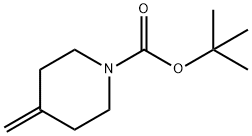
159635-49-1
247 suppliers
$5.00/250mg
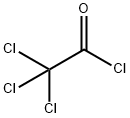
76-02-8
250 suppliers
$59.70/50g
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
169 suppliers
$8.00/250mg

159635-49-1
247 suppliers
$5.00/250mg
![tert-butyl 2-oxo-7-azaspiro[3.5]nonane-7-carboxylate](/CAS/GIF/203661-69-2.gif)
203661-69-2
169 suppliers
$8.00/250mg