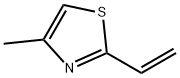
2-VINYL-4-METHYLTHIAZOLE, 99+% synthesis
- Product Name:2-VINYL-4-METHYLTHIAZOLE, 99+%
- CAS Number:45534-10-9
- Molecular formula:C6H7NS
- Molecular Weight:125.19
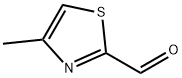
13750-68-0
108 suppliers
$20.00/250mg
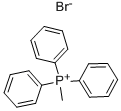
1779-49-3
612 suppliers
$5.00/10g

45534-10-9
15 suppliers
inquiry
Yield:45534-10-9 54%
Reaction Conditions:
Stage #1: Methyltriphenylphosphonium bromidewith n-butyllithium in tetrahydrofuran;hexane at 0 - 5; for 1 h;Inert atmosphere;
Stage #2: 4-methyl-thiazole-2-carbaldehyde in tetrahydrofuran;hexane at 0 - 20;
Steps:
5.5.3 5.3 Synthesis of 2-ethenyl-4-methyl-1 ,3-thiazole a5-3.
In a three-necked round bottom flask under argon, BuLi (1 .6 M solution in hexane, 3.43 ml, 5.5 mmol, 1.1 eq) is slowly added to methyl(triphenyl)phosphonium bromide (1.79 g, 5 mmol, 1 eq) in THF (15 ml) without exceeding 5 °C. After 1 h under stirring at 0°C, 4-methyl-1 ,3-thiazole-2-carbaldehyde (635 mg, 5 mmol, 1 eq) is added. The reaction mixture is allowed to return to ambient temperature and stirred overnight. The reaction is quenched with Rochelle salt (potassium sodium tartrate) (2 g) and 3 drops of water. The mixture is diluted with diethyl ether and dried over magnesium sulfate. Solvents are removed under vacuum and the crude product is distilled (+/- 60°C; 10"2 Bar) to afford 335 mg of 2-ethenyl-4-methyl-1 ,3-thiazole a5-3 as a clear oil.Yield: 54 %.H NMR (CDCI3 , 400MHz) δ: 6.87 (dd, J-| = 17.5 Hz, J2 = 10.9 Hz, 1 H), 6.78 (s, 1 H), 6.01 (d, J = 17.5 Hz, 1 H), 5.50 (d, J = 10.9 Hz, 1 H), 2.44 (s, 3 H).
References:
WO2013/53725,2013,A1 Location in patent:Page/Page column 52
333385-00-5
6 suppliers
inquiry

45534-10-9
15 suppliers
inquiry