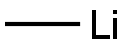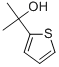
2-(Thiophen-2-yl)propan-2-ol synthesis
- Product Name:2-(Thiophen-2-yl)propan-2-ol
- CAS Number:5331-62-4
- Molecular formula:C7H10OS
- Molecular Weight:142.22
Yield:5331-62-4 84.6%
Reaction Conditions:
Stage #1: thiophenewith n-butyllithium in tetrahydrofuran at -78; for 1 h;
Stage #2: propan-2-one in tetrahydrofuran;
Steps:
30.1 Step 1
To a 500 mL three necked flask was added 100 mL THF and thiophene (17.0 g, 0.2 mol, 1.0 eq) at -78 °C, then n-Butyllithium (80 mL, 0.2 mol, 1 eq) were added dropwise to the mixture, the reaction mixture was stirred for 1 h under -78 °C. Then Acetone (16.8 g, 0.2 mol, 1 eq) were added dropwise to the mixture. TLC (petroleum ether: EA=10:l, Rf= 0.4) showed the reaction was having a new point. The reaction was quenched with NH4C1 (100 mL), and extracted with EA (100*3). The layer was dried over Na2SO4 and filtered. The mixture was cooled and concentrated in vacuum and e resulting product was purified by column chromatography on silica gel to yield the product 30A (21 g) as a light oil. Yield: 84.6%.
References:
WO2022/76383,2022,A1 Location in patent:Paragraph 00282
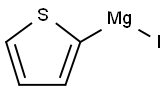
89180-57-4
0 suppliers
inquiry
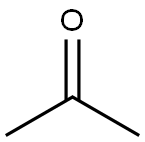
67-64-1
0 suppliers
$19.10/10ml
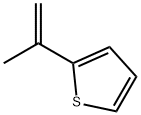
30616-73-0
13 suppliers
inquiry

5331-62-4
12 suppliers
inquiry