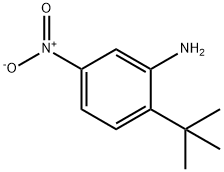
2-tert-Butyl-5-nitro-phenylamine synthesis
- Product Name:2-tert-Butyl-5-nitro-phenylamine
- CAS Number:103392-84-3
- Molecular formula:C10H14N2O2
- Molecular Weight:194.23
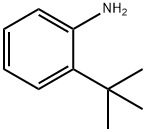
6310-21-0
216 suppliers
$6.00/1g

103392-84-3
28 suppliers
inquiry
Yield: 94%
Reaction Conditions:
with sulfuric acid;potassium nitrate at 5 - 20; for 1.5 h;
Steps:
9.1
2-(tert-butyl)aniline (10 g, 67.01 mmol) was slowly added dropwise to sulfuric acid (63.8 mL, 670.09 mmol) while the mixture was maintained at 10°C or below. Then, potassium nitrate (6.77 g, 67.01 mmol) was slowly added thereto while the mixture was maintained at 10°C or below. The reaction mixture was stirred at 5°C for 30 min, and then at room temperature for 1 hr. The reaction mixture was poured into ice (about 500 g), and the mixture was extracted with diethyl ether. The organic layer was washed with brine, and dried over magnesium sulfate, and the solvent was evaporated under reduced pressure. The obtained residue was purified by silica gel column chromatography (solvent; 20% ethyl acetate/hexane), and crystallized from hexane to give 2-(tert-butyl)-5-nitroaniline (12.26 g, 63.1 mmol, 94%) as a pale yellow solid.
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;YAMAMOTO, SATOSHI;SHIRAI, JUNYA;WATANABE, HIROYUKI;FUKUMOTO, SHOJI;ODA, TSUNEO;TOKUHARA, HIDEKAZU;TOMATA, YOSHIHIDE;ISHII, NAOKI;TAWADA, MICHIKO;KOUNO, MITSUNORI;OCHIDA, ATSUKO;IMADA, TAKASHI;FUKASE, YOSHIYUKI;YUKAWA, TOMOYA TW2016/2105, 2016, A Location in patent:Paragraph 0575
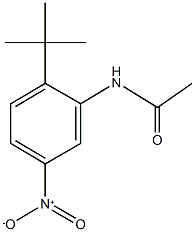
342045-15-2
2 suppliers
inquiry

103392-84-3
28 suppliers
inquiry
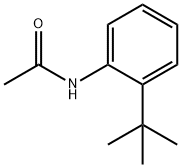
7402-70-2
9 suppliers
inquiry

103392-84-3
28 suppliers
inquiry
