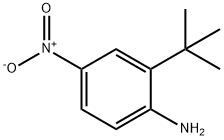
2-tert-butyl-4-nitrobenzenamine synthesis
- Product Name:2-tert-butyl-4-nitrobenzenamine
- CAS Number:59255-98-0
- Molecular formula:C10H14N2O2
- Molecular Weight:194.23
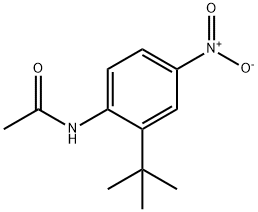
815586-43-7
1 suppliers
inquiry

59255-98-0
6 suppliers
inquiry
Yield:59255-98-0 100%
Reaction Conditions:
with hydrogen bromide in water at 110; for 3 h;
Steps:
3.3
N-(2-tert-butyl-4-nitrophenyl)acetamide (840 mg, 3.57 mmol) was dissolved in concentrated HBr (48%, 20 mL). The resulting solution was heated at 110° C. for 3 h. The reaction mixture was cooled to rt, diluted with EtOAc, and poured into sat. NaHCO3 solution. The organic phase was extracted with sat. NaHCO3 solution and brine, dried over Na2SO4, filtered and concentrated to give the desired product as a yellow-green oil (710 mg, >100%). 1H NMR (400 MHz, CD3OD) δ: 7.52 (d, 1H), 7.40 (dd, 1H), 7.33 (d, 1H), and 1.42 (s, 9H).
References:
US2006/160803,2006,A1 Location in patent:Page/Page column 137
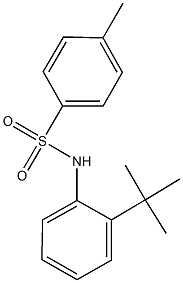
6683-78-9
2 suppliers
inquiry

59255-98-0
6 suppliers
inquiry
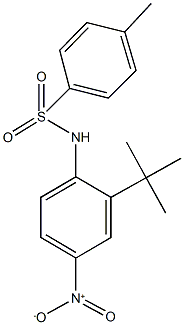
6683-79-0
2 suppliers
inquiry

59255-98-0
6 suppliers
inquiry
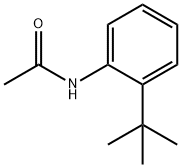
7402-70-2
10 suppliers
inquiry

59255-98-0
6 suppliers
inquiry
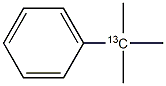
253185-04-5
0 suppliers
inquiry

59255-98-0
6 suppliers
inquiry