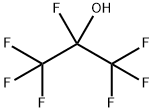
2-Propanol, 1,1,1,2,3,3,3-heptafluoro- synthesis
- Product Name:2-Propanol, 1,1,1,2,3,3,3-heptafluoro-
- CAS Number:24427-67-6
- Molecular formula:C3HF7O
- Molecular Weight:186.03
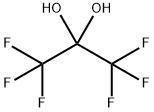
677-71-4
2 suppliers
inquiry

24427-67-6
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen fluoride at -20 - 60;Product distribution / selectivity;
Steps:
1
As to material of the dehydration apparatus, a fluororesin or polyethylene was used for all of the parts with which hexafluoroacetone, hexafluoroacetone trihydrate and anhydrous hydrogen fluoride are brought into contact. As the distillation pot, a polytetrafluoroethylene container of 10cm diameter x 15 cm height was used. As the distillation column, there was used a PFA column of 3cm diameter x 45cm height packed by a length of 35cm with polyethylene Raschig rings (4mm diameter x 4mm length) as a packing material. As the condenser, there was used a PFA cylindrical container of 10cm diameter x 15cm height equipped in the inside with a coiled tube to make a refrigerant to flow. As a receiver for an effluent from the column top, a 500ml PFA container was used. After putting 220g of HFA·3W into the distillation pot, it was cooled with a dry ice/acetone bath to solidify it. Then, 240g of anhydrous hydrogen fluoride was weighed and put thereinto. Under a cooled condition (-20°C), it was set on the distillation column. It was allowed to stand still as it was until room temperature. Then, heating was started with a water bath and then an oil bath. During this, a conspicuous generation of heat in the distillation pot by the mixing was not observed. As the heating was continued, reflux was observed at an upper part of the distillation column. Then, temperature of the reflux portion started to become lower than boiling point (19.5°C) of hydrogen fluoride, and reflux of HFA·HF was confirmed. Upon this, it was started to take the reflux liquid out, while securing reflux to maintain temperature of the column top at around 19.5°C, boiling point of hydrogen fluoride. The reflux liquid (HFA·nHF) taken out was received in the receiver cooled with ice, and the volume was recorded over time. At the time when a suitable amount was collected, the container was changed, followed by measuring the volume and the mass, putting into a storage container made of polyethylene, and storing with sealing in a refrigerator. Since the column bottom temperature increases with lowering of the hydrogen fluoride concentration at the column bottom portion by the progress of the distillation, the oil bath temperature was adjusted to always have a temperature difference of 20°C or greater between the column top and the column bottom. Temperature of the column bottom showed on occasions temperature of the gas phase portion of the distillation pot due to decrease of the amount of the interior content (It was the same in the following.). Temperatures of the column bottom portion and the distillation column increased gradually, but temperature of the column top was almost constantly maintained at 20°C to maintain a suitable reflux. At the time when the column bottom temperature showed 60°C, the distillation was stopped. After the distillation, the reflux liquid (column top effluent) recovered from the column top was 252g (the molar ratio n of HFA·nHF: 4.3, yield of hexafluoroacetone: 99%), and the column bottom liquid was a hydrogen fluoride aqueous solution (71 mass %) of 188g. Existence of water was not confirmed by measuring water content of the reflux liquid by Karl Fischer's method. Hexafluoroacetone was not detected in the column bottom liquid.
References:
EP2189437,2010,A1 Location in patent:Page/Page column 6
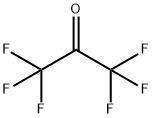
684-16-2
0 suppliers
$120.00/5g

24427-67-6
0 suppliers
inquiry