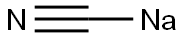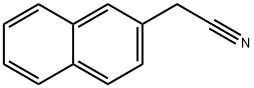
2-Naphthylacetonitrile synthesis
- Product Name:2-Naphthylacetonitrile
- CAS Number:7498-57-9
- Molecular formula:C12H9N
- Molecular Weight:167.21
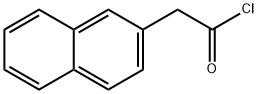
37859-25-9
86 suppliers
$28.00/250mg

7498-57-9
160 suppliers
$6.00/1g
Yield:7498-57-9 60.38 g
Reaction Conditions:
with sulfolane;SULFAMIDE in toluene at 95 - 105; for 4 h;Inert atmosphere;Temperature;Time;
Steps:
2-6
In another nitrogen-substituted reactor were added sulfamide (46.49 g) (1.2 mole ratio to 2-naphthylacetic acid), an inorganic additive (74.99 g) (1 weight ratio to 2-naphthylacetic acid), and sulfolane (263 mL) (3.5 volume ratio to 2-naphthylacetic acid), and the mixture was heated (preparation of reaction starting material 2). The reaction starting material 1 containing 2-naphthylacetyl chloride was added dropwise to the reaction starting material 2 at 95° C.-105° over 18 min. The instrument used for preparing the reaction starting material 1 was washed with toluene (7.5 mL) (0.1 volume ratio to 2-naphthylacetic acid), the obtained solution was further added to the reaction starting material 2, and the mixture was reacted at 95° C.-105° C. for 4 hr. The reaction mixture was analyzed by HPLC, and the disappearance of the starting materials was confirmed. Then, the mixture was cooled to 20° C.-30° C., water (300 mL) (4 volume ratio to 2-naphthylacetic acid) and toluene (300 mL) (4 volume ratio to 2-naphthylacetic acid) were added, the mixture was stirred, and the aqueous layer was discarded. The remaining organic layer was washed with 10 wt % potassium carbonate aqueous solution (225.08 g) (3 weight ratio to 2-naphthylacetic acid) and water (150 mL) (2 volume ratio to 2-naphthylacetic acid). The obtained organic layer was concentrated, methanol (525 mL) (7 volume ratio to 2-naphthylacetic acid) was added to the concentrated residue, and the mixture was concentrated again. Furthermore, methanol was added to the obtained concentrated residue to adjust the liquid amount to 525 mL. Activated carbon (1.52 g) (0.02 weight ratio to 2-naphthylacetic acid) was added, and the mixture was stirred at 50° C.-60° C. and filtered. The obtained filtration residue was washed with methanol (75 mL) (1 volume ratio to 2-naphthylacetic acid). The obtained filtrate and washing solution were cooled to 5° C.-15° C., water (300 mL) (4 volume ratio to 2-naphthylacetic acid) was added, and the mixture was stirred. The precipitated 2-naphthylacetonitrile was collected by filtration, and the obtained wet crystals were dried to obtain 2-naphthylacetonitrile (60.38 g) (purity 99.85 area %) as a solid.
References:
US2022/9880,2022,A1 Location in patent:Paragraph 0202-0204; 0209-0212; 0217-0220; 0224; 0226
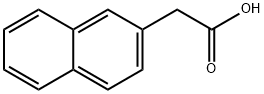
581-96-4
231 suppliers
$6.00/5g

7498-57-9
160 suppliers
$6.00/1g
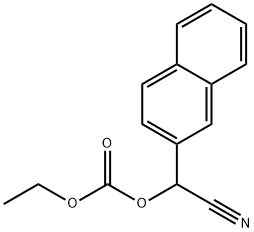
959842-57-0
0 suppliers
inquiry

7498-57-9
160 suppliers
$6.00/1g

939-26-4
311 suppliers
$5.00/1g

7498-57-9
160 suppliers
$6.00/1g