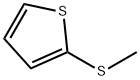
2-(METHYLTHIO)THIOPHENE synthesis
- Product Name:2-(METHYLTHIO)THIOPHENE
- CAS Number:5780-36-9
- Molecular formula:C5H6S2
- Molecular Weight:130.23
188290-36-0
1 suppliers
inquiry
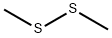
624-92-0
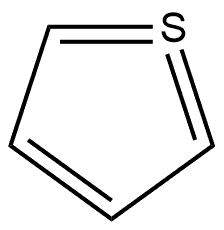
332 suppliers
$20.62/5 mL
554-14-3
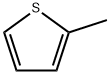
259 suppliers
$6.00/5g

5780-36-9
101 suppliers
$36.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield: 17% , 11% , 6%
Reaction Conditions:
with highly siliceous zeolite HZSM-5 in a hydrogen form (SiO2/Al2O3 = 34) (Ssp 500 m2/g) at 180; for 0.00388889 h;
Steps:
We used as catalyst an industrial sample of highlysiliceous zeolite HZSM-5 in a hydrogen form (SiO2/Al2O3 = 34) (Ssp 500 m2/g) calcined before use in astream of dry air for 5 h at 530°. The substances usedin the study were reagents of “pure” grade.The catalysis experiments were carried out atatmospheric pressure in a flow reactor with a fixedcatalyst bed, catalyst grains of 0.25-0.5 mm, underconditions excluding diffusion difficulties. Each runwas performed with the use of fresh catalyst. Thesystem was totally under temperature control. Into thesaturators filled with dimethyl disulfide and thiopheneplaced in thermostats helium was passed from a gascylinder. After mixing the gas saturated with dimethyldisulfide and thiophene was passed into the reactorwith catalyst heated at the desired temperature. The gassampling was carried out every 45 min within ~2 h. Thehigh boiling products were condensed in a cooledreceiver.Reaction products were identified by GC-MS on aninstrument Agilent 7000 GC/MS Triple Quad equippedwith a capillary column (30 m × 0.25 mm),stationary phase HP-5MS. The quantitative analysiswas carried out on a chromatograph LKhM-8MDequipped with a katharometer, column 2 m × 3 mm,stationary phase XE-60 on Chromaton AW-LMCS,carrier gas helium. Contact time is equal to the ratio ofthe catalyst volume (cm3) to the gas flow rate (cm3/s)at room temperature and atmospheric pressure. Theconversion of thiophene and dimethyl disulfide, andalso the yields of the reaction products were calculatedfrom the concentration of the formed product withrespect to the initial concentration of the substarte(mol %), and the selectivity of product formation was evaluated from the ratio of its yield to the substrate conversion.
References:
Mashkina;Khairulina [Russian Journal of Organic Chemistry,2015,vol. 51,# 2,p. 217 - 220][Zh. Org. Khim.,2015,vol. 51,# 2,p. 217 - 220,4]

188290-36-0
1 suppliers
inquiry
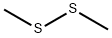
624-92-0
332 suppliers
$20.62/5 mL

5780-36-9
101 suppliers
$36.00/1g
3437-95-4
244 suppliers
$6.00/5g
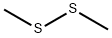
624-92-0
332 suppliers
$20.62/5 mL

5780-36-9
101 suppliers
$36.00/1g

188290-36-0
1 suppliers
inquiry
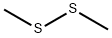
624-92-0
332 suppliers
$20.62/5 mL

554-14-3
259 suppliers
$6.00/5g
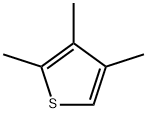
1795-04-6
19 suppliers
inquiry

5780-36-9
101 suppliers
$36.00/1g
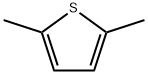
638-02-8
255 suppliers
$10.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

188290-36-0
1 suppliers
inquiry
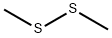
624-92-0
332 suppliers
$20.62/5 mL

5780-36-9
101 suppliers
$36.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry