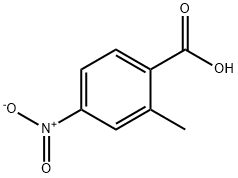
2-Methyl-4-nitrobenzoic acid synthesis
- Product Name:2-Methyl-4-nitrobenzoic acid
- CAS Number:1975-51-5
- Molecular formula:C8H7NO4
- Molecular Weight:181.15
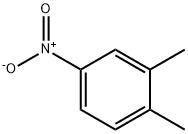
99-51-4
18 suppliers
$13.00/25g

1975-51-5
312 suppliers
$6.00/1g
Yield:1975-51-5 72%
Reaction Conditions:
with N-hydroxyphthalimide;cobalt(II) chloride hexahydrate;nitric acid;manganese (II) acetate tetrahydrate under 760.051 Torr; for 12 h;Reflux;Reagent/catalyst;Concentration;
Steps:
Preparation of 2-methyl-4-nitrobenzoic acid
Preparation of 2-methyl-4-nitrobenzoic acid4-Nitro-o-xylene (0.15 g, 1 mmol), NHPI (0.05 g,0.3 mmol), nitric acid (40 %; 1.26 g, 8 mmol),CoCl2 ·6H2O (7.1 mg, 0.03 mmol), Mn(OAc)2 ·4H2O(7.1 mg, 0.03 mmol) and a phase transfer catalyst(0.04 mmol) were added to a 50 mL three-neckedround bottomed flask equipped with a magnetic stirrerand a reflux condenser. The mixture was heated underreflux for 12 h at normal pressure. After the reactioncompletion the mixture was cooled to room temperatureand washed with 10 mL of water. The aqueousphase was extracted with ethyl acetate (3 × 5 mL)and the obtained organic phase was dried with anhydroussodium sulfate. Ethyl acetate was removedfrom the organic layer by rotary evaporation and thecrude products were determined by the HPLC analysisbased on the internal standard (2,4-dinitrotoluene).The crude products were re-crystallized (EtOH/H2Or = 3 : 1) to give pure 2-methyl-4-nitrobenzoic acid(0.13 g, 72 %) in form of white needle crystals; m.p.:151-152C. IR, ν/cm-1, (KBr): 1702 cm-1.1H NMR(CDCl3, 500 MHz), δ: 2.79 (s, 3H, CH3 ), 8.13-8.17(m, 2H, Ar), 8.21 (d, 1H, J = 6.8 Hz, Ar). Anotherproduct is the 4-nitrophthalic acid which is separatedby column chromatography from ethyl acetate/hexane(r = 1 : 5) in form of light colored needle crystals;m.p.:163C. IR, ν/cm-1, (KBr): 1730 cm-1,1670 cm-1. 1H NMR (CDCl3, 500 MHz), δ: 7.86 (d,J = 8.6 Hz, 1H, Ar), 8.36 (dd, J1 = 8.6Hz, J2 = 2.0Hz,1H, Ar), 8.42 (d, J = 2.0 Hz, 1H, Ar) (Huntress et al.,1936).
References:
Wei, Song-Bo;Tang, Bo;Peng, Xin-Hua [Chemical Papers,2015,vol. 69,# 4,p. 580 - 585]
99584-16-4
13 suppliers
inquiry

1975-51-5
312 suppliers
$6.00/1g
89001-53-6
91 suppliers
$19.00/1g

1975-51-5
312 suppliers
$6.00/1g
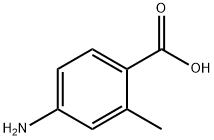
2486-75-1
245 suppliers
$8.00/1g

1975-51-5
312 suppliers
$6.00/1g
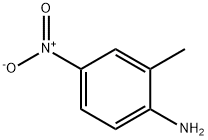
99-52-5
291 suppliers
$5.00/5 g

1975-51-5
312 suppliers
$6.00/1g