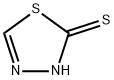
2-Mercapto-1,3,4-thiadiazol synthesis
- Product Name:2-Mercapto-1,3,4-thiadiazol
- CAS Number:18686-82-3
- Molecular formula:C2H2N2S2
- Molecular Weight:118.18
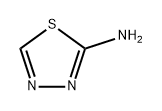
4005-51-0
290 suppliers
$5.00/10g

18686-82-3
243 suppliers
$9.00/5g
Yield:-
Reaction Conditions:
with hydrogen bromide;copper;sodium nitrite
Steps:
2-Mercapto-1,3,4-thiadiazole
2-Mercapto-1,3,4-thiadiazole The procedure of J. Goerdeler, J. Ohm and O. Tegtmeyer, Berichte 89, 1534 (1956) was followed. To a solution of 160 ml. of 48% hydrobromic acid and 100 mg. of powdered copper at -7° was added slowly with alternation 13.6 g. (0.1 mole) of 2-amino-1,3,4-thiadiazole (Eastman) and 32 g. of sodium nitrite in small portions over a period of one-half hour. The mixture was stirred for 1 1/2 hours at 0° and for 1 hour at room temperature. The mixture was then neutralized with 50% potassium hydroxide to pH 9.5. The mixture was filtered and the filtrate was continuously extracted with ether for 6 hours. The ether was evaporated to 15 mm (25°) to a solid which was dissolved in 40 ml. of ethyl alcohol and treated with 5 g. of thiourea.
References:
Bristol-Myers Company US3946000, 1976, A