2-Iodobenzyl bromide synthesis
- Product Name:2-Iodobenzyl bromide
- CAS Number:40400-13-3
- Molecular formula:C7H6BrI
- Molecular Weight:296.93
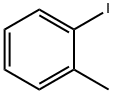
615-37-2
296 suppliers
$5.00/5g

40400-13-3
179 suppliers
$21.00/1G
Yield:40400-13-3 69%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethaneReflux;
Steps:
1-(bromomethyl)-2-iodobenzene
A mixture of l-iodo-2-methylbenzene (0.5 g, 2.3 mmol), N-bromosuccinimide (0.7 g, 3.7 mmol) and 2,2'-azobisisobutyronitrile (0.02 g, 0.1 mmol) in tetrachloromethane (10 mL) was heated at reflux until the reaction was completed (tic control). After cooled down to the ambient temperature the reaction mixture was quenched with water and water layer was extracted with dichloromethane. Combined organic phases were washed with sodium hydrocarbonate, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel; hexane/ethyl acetate 9: 1) to give l-(bromomethyl)-2-iodobenzene (0.5 g) as yellowish oil; yield 69%.
References:
SELVITA S.A.;RZYMSKI, Tomasz;MILIK, Mariusz;BRZOZKA, Krzysztof;FABRITIUS, Charles-Henry;KUCWAJ-BRYSZ, Katarzyna;KULESZA, Urszula;WINCZA, Ewelina;DREAS, Agnieszka;GALEZOWSKI, Michal WO2017/68064, 2017, A1 Location in patent:Page/Page column 188; 189
5159-41-1
223 suppliers
$8.00/5g

40400-13-3
179 suppliers
$21.00/1G
18698-96-9
251 suppliers
$5.00/250mg

40400-13-3
179 suppliers
$21.00/1G
624-31-7
438 suppliers
$9.00/25g

40400-13-3
179 suppliers
$21.00/1G