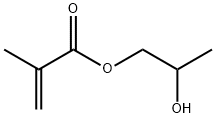
2-Hydroxypropyl methacrylate synthesis
- Product Name:2-Hydroxypropyl methacrylate
- CAS Number:923-26-2
- Molecular formula:C7H12O3
- Molecular Weight:144.17
Yield:-
Reaction Conditions:
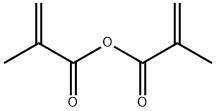
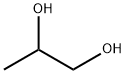
Stage #1:propylene glycol with pyridine;dmap in tetrahydrofuran at 23; for 0.5 h;
Stage #2:methacryloyl anhydride in tetrahydrofuran at 5 - 23; for 1 h;
Steps:
1
49.36 parts of the compound represented by the formula (I-2-a), 135 parts of tetrahydrofuran, 15.39 parts of pyridine and 0.79 parts of dimethylaminopyridine were added and stirred at 23 ° C. for 30 minutes. The obtained mixed solution was cooled to 5 ° C., and 20 parts of the compound represented by the formula (I-2-b) Was added over 30 minutes. Thereafter, the mixture was further stirred at 23 ° C. for 1 hour. 380 parts of ethyl acetate and 171 parts of 5% hydrochloric acid were charged in the obtained reaction product, and the mixture was stirred at 23 ° C. for 30 minutes, and the mixture was allowed to stand and was separated to wash the organic layer. 77 parts of ion-exchanged water was charged to the recovered organic layer The mixture was stirred at 23 ° C. for 30 minutes, allowed to stand and separated, and the organic layer was washed with water. To the recovered organic layer,And 54 parts of a 10% potassium carbonate aqueous solution were charged and stirred at 23 ° C. for 30 minutes, and the mixture was allowed to stand still and the organic layer was washed with water. To the recovered organic layer, 115 parts of deionized water was added And the mixture was stirred at 23 ° C. for 30 minutes, and the organic layer was washed with water by allowing to stand and separating. This washing operation was repeated four times. The recovered organic layer was concentrated. The concentrated mass thus obtained was applied to a column (Kanto Chemical Co., Ltd. Silica gel 60 N (spherical, neutral) 100-210 μm developing solvent: n-Hep Tan / ethyl acetate = 1/1), the recovered organic layer was concentrated to obtain 38.48 parts of a compound represented by the formula (I-2-c).
References:
Sumitomo Chemical Co., Ltd.;Masuyama, Tatsuro;Ichikawa, Koji JP2017/105770, 2017, A Location in patent:Paragraph 0222
57-55-6
1592 suppliers
$5.00/1g
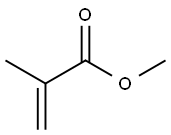
80-62-6
624 suppliers
$18.00/25mL

923-26-2
65 suppliers
inquiry
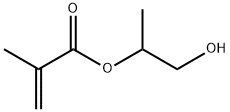
4664-49-7
12 suppliers
inquiry