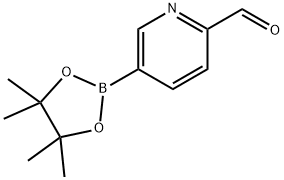
2-FORMYLPYRIDINE-5-BORONIC ACID PINACOLATE synthesis
- Product Name:2-FORMYLPYRIDINE-5-BORONIC ACID PINACOLATE
- CAS Number:1073354-14-9
- Molecular formula:C12H16BNO3
- Molecular Weight:233.07
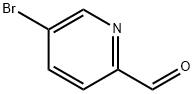
31181-90-5
345 suppliers
$5.00/250mg
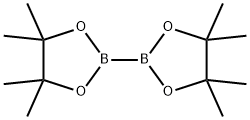
73183-34-3
591 suppliers
$6.00/5g

1073354-14-9
73 suppliers
$65.00/100 mg
Yield: 15.2g
Reaction Conditions:
Stage #1:5-bromo-pyridine-2-carbaldehyde;bis(pinacol)diborane with potassium acetate in tetrahydrofuran at 20; for 0.5 h;
Stage #2: with tris-(dibenzylideneacetone)dipalladium(0);XPhos in tetrahydrofuran at 20 - 75; for 5 h;
Steps:
195.a Example 195 3-(3-(5-(Pyrimidin-2-yl)pyridin-2-yl)-1H-pyrazol-5-yl)pyrrolidine-1-carbonitrile
Step a. To a stirred solution of 5-bromo-2-pyridinecarboxaldehyde (5.00 g, 26.9 mmol) in THE (50 ml) was added bis(pinacolato)diboron (10.23 g, 40.3 mmol) and potassium acetate (7.91 g, 80.6 mmol) at rt. The reaction mixture was degassed for 30 min before addition of Pd2(dba)3 (1.230 g, 1.34 mmol) and X-phos (1.28 g, 26.88 mmol) at rt. The reaction mixture was heated at 75° C. for 5 h. The resulting reaction mixture was cooled to rt, filtered through celite bed and washed with EtOAc (2×100 ml). The combined filtrate was concentrated under reduced pressure yielding 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)picolinaldehyde (15.2 g). This material was directly used for the next step without further purification. MS: ES+152 (M-82)
References:
MISSION THERAPEUTICS LIMITED;JONES, Alison;KEMP, Mark Ian;STOCKLEY, Martin Lee;WOODROW, Michael D. US2020/331888, 2020, A1 Location in patent:Paragraph 0697-0698
