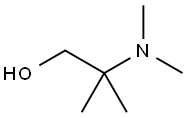
2-DIMETHYLAMINO-2-METHYL-1-PROPANOL synthesis
- Product Name:2-DIMETHYLAMINO-2-METHYL-1-PROPANOL
- CAS Number:7005-47-2
- Molecular formula:C6H15NO
- Molecular Weight:117.19
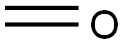
50-00-0
892 suppliers
$10.00/25g
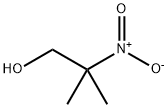
76-39-1
70 suppliers
$181.00/250mg

7005-47-2
91 suppliers
$21.00/5mL
Yield:-
Reaction Conditions:
Stage #1:2-nitro-2-methylpropanol with hydrogen
Stage #2:formaldehyd
Steps:
1
Conversion of NMP to DMAMP. NMP is converted to DMAMP by a stepwise reduction with hydrogen to form 2-amino-2-methyl- 1 -propanediol (AMP). The AMP is not isolated but reacted in situ with the excess formaldehyde (from step 1) so that DMAMP is continuously being formed throughout the hydrogenation. Upon completion, and depending on the temperature during the NMP feed, the temperature is increased to promote methylation. GC scans of in-process samples show multiple peaks. These various compounds are not impurities but rather intermediates, including the monooxazolidine of AMP, the monomethylated AMP, and the monooxazolidine of MM AMP. After 1 hour at elevated temperature, a methyl formcel trim is slowly fed to the autoclave. After the methyl formcel trim, the reactor is held at temperature for an additional hour to complete the methylation.For higher yield and product purity, the NMP feed is preferably carried out at the lowest practical temperature (see reaction No.1 in the table below). To simplify the process, in reaction No.2, all autoclave steps are carried out at 100°C. This reaction also works well although, as would be expected at higher nitroalcohol feed temperatures, there is some yield loss, indicated by the higher level of Ν,Ν-dimethylisopropylamine (DMIPA). For further simplification of the process, the NMP may be fed at 65°C and cooling water shut off at different points to allow the reaction exotherm to increase the temperature prior to the methyl formcel trim (to complete the methylation).GC-MS CI [M+H] = 118 with retention time 8.07 min, confirming the formation of DMAMP. GC analysis indicates conversion to DMAMP of between 88 % and 97 , depending on the specific reaction conditions used.
References:
ANGUS CHEMICAL COMPANY;MOORE, David W.;SWEDO, Raymond;PEERA, Asghar A. WO2012/44508, 2012, A1 Location in patent:Page/Page column 12
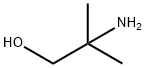
124-68-5
555 suppliers
$4.76/25g

7005-47-2
91 suppliers
$21.00/5mL