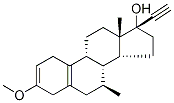
2-Dehydro-3-Methoxy Tibolone synthesis
- Product Name:2-Dehydro-3-Methoxy Tibolone
- CAS Number:15506-05-5
- Molecular formula:C22H30O2
- Molecular Weight:326.47
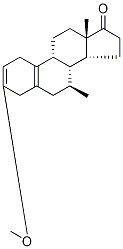
5210-25-3
10 suppliers
$120.00/10mg
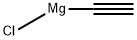
65032-27-1
89 suppliers
$72.40/100ml

15506-05-5
3 suppliers
inquiry
Yield:15506-05-5 82%
Reaction Conditions:
in tetrahydrofuran at 20 - 35; for 0.5 h;
Steps:
7A Example 7A; Stage 1
Compound (9A) (30.0 g) was dissolved in tetrahydrofuran (90 mL) at ambient temperature under nitrogen. This solution was added to a vacuum degassed solution of ethynyl magnesium chloride in tetrahydrofuran (325 mL, d = 0.921, MOLARITY 0. 48 M) at 25-30 C under nitrogen over at least 30 minutes. The reaction mixture was stirred at 25-30 C until completed, as assessed by HPLC. The reaction mixture was transferred under nitrogen to a 13 % w/w solution of ammonium chloride in water (300 mL) at A rate to maintain the temperature of the quench mixture between 20-30 C. Celite (6.0 g) was then added to the biphasic reaction mixture which was then stirred for 30 minutes at 302 C. The resultant slurry was filtered and the upper organic layer was separated. The organic layer was washed with 30 % w/w sodium chloride solution (150 mL). The organic solution was concentrated under reduced pressure to 6 volumes based on the compound (9A) input weight. Deionised water was added dropwise to complete the precipitation and the resultant slurry was cooled to 0-5 C and stirred for at least one hour. The solid was collected by filtration. The filter cake was washed with a 1: 1 mixture of THF/WATER and finally with water and pyridine solution. The solid was dried in the vacuum oven at 30-35 C under vacuum until the water content was below 5% as judged by Karl Fischer titration. STAGE 2 The crude dried solid (33.9 g) from Stage 1 was dissolved in a mixture of methanol (160 mL) and pyridine (0.38 mL) at 552 C under nitrogen to form a clear solution. Water (14 mL) was added dropwise at 55~2°C and the mixture cooled. Crystallisation occurred at 50~5°C. The slurry was cooled to 0 to 5 C and stirred at this temperature for at least one hour. The solid was isolated by filtration and dried at 30-35°C under vacuum to constant weight (yield : 82%).
References:
WO2004/78774,2004,A1 Location in patent:Page 38-39

5210-25-3
10 suppliers
$120.00/10mg
1066-26-8
98 suppliers
$48.00/50g

15506-05-5
3 suppliers
inquiry
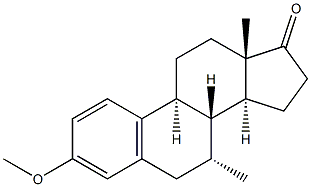
10449-00-0
0 suppliers
inquiry

15506-05-5
3 suppliers
inquiry
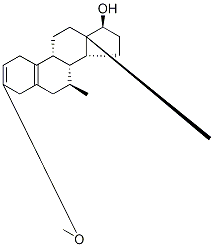
15506-02-2
9 suppliers
$2440.00/200mg

15506-05-5
3 suppliers
inquiry
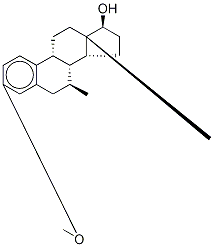
15506-01-1
7 suppliers
$650.00/20mg

15506-05-5
3 suppliers
inquiry