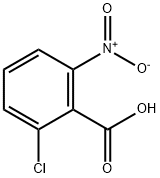
2-Chloro-6-nitrobenzoic acid synthesis
- Product Name:2-Chloro-6-nitrobenzoic acid
- CAS Number:5344-49-0
- Molecular formula:C7H4ClNO4
- Molecular Weight:201.56

50907-57-8
80 suppliers
$23.00/250mg

5344-49-0
152 suppliers
$10.00/5g
Yield:5344-49-0 78%
Reaction Conditions:
with water;nitric acid in 1,2-dichloro-benzene at 100 - 140; under 7500.75 Torr; for 8.66667 h;Product distribution / selectivity;
Steps:
4
The organic phase from example 2 was heated to 100° C., and 1,2-dichlorobenzene was distilled off at 15 mbar over 2 hours to a residual content of 4% by weight.400 mol % (based on 2-chloro-6-nitrotoluene used) of 65% strength by weight nitric acid were initially introduced into an autoclave and heated to 140° C. The benzyl alcohol, largely freed from the solvent, was metered in uniformly over the course of 8.5 hours. During this operation, the pressure in the autoclave was kept constant at 10 bar. Escaping nitric acid and 1,2-dichlorobenzene was not replaced. After the total amount of benzyl alcohol had been pumped in, the mixture was after-stirred for 10 minutes, cooled to 100° C. and decompressed.Aqueous nitric acid was distilled off with a gentle vacuum, and the residue which remained was dissolved with 400 g of 1,2-dichlorobenzene.Analysis of the mixture revealed 1.44 mol % of unreacted 2-chloro-6-nitrobenzyl alcohol, 0.26 mol % of 2-chloro-6-nitrobenzaldehyde and 90 mol % of 2-chloro-6-nitrobenzoic acid.The mixture was cooled to 25° C. over the course of 4 hours. The precipitated 2-chloro-6-nitrobenzoic acid was filtered off and washed with a small amount of cold 1,2-dichlorobenzene. Drying gave pure 2-chloro-6-nitrobenzoic acid in 78% yield (based on 2-chloro-6-nitrotoluene used).
References:
US6933406,2005,B1 Location in patent:Page/Page column 5
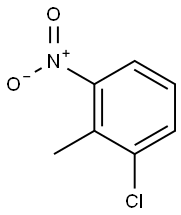
83-42-1
268 suppliers
$9.00/5g

5344-49-0
152 suppliers
$10.00/5g
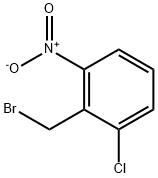
56433-01-3
70 suppliers
inquiry

5344-49-0
152 suppliers
$10.00/5g
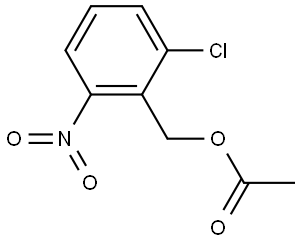
919076-97-4
0 suppliers
inquiry

5344-49-0
152 suppliers
$10.00/5g
88-72-2
263 suppliers
$15.00/5g

5344-49-0
152 suppliers
$10.00/5g