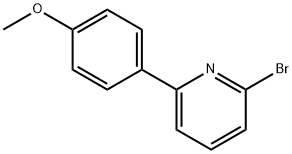
2-broMo-6-(4-Methoxyphenyl)pyridine synthesis
- Product Name:2-broMo-6-(4-Methoxyphenyl)pyridine
- CAS Number:193344-39-7
- Molecular formula:C12H10BrNO
- Molecular Weight:264.12
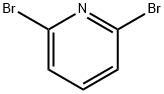
626-05-1
504 suppliers
$9.00/10g
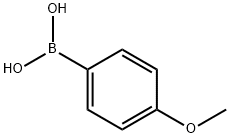
5720-07-0
464 suppliers
$6.00/5g
21172-80-5
3 suppliers
inquiry

193344-39-7
9 suppliers
inquiry
Yield:193344-39-7 77 %Spectr. ,21172-80-5 7 %Spectr.
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;water at 30; for 28 h;chemoselective reaction;Suzuki-Miyaura Coupling;
Steps:
Suzuki-Miyaura coupling reaction of 2,6-dibromopyridine (5) with 4-methoxyphenylboronic acid using PS-PdMC as catalyst (Table 1, Entries 2 and 3)
A mixture of 2,6-dibromopyridine (5) (7.6 mg, 0.032 mmol), 4-methoxyphenylboronic acid (9.7 mg, 0.064 mmol, 2.0 eq.), PS-PdMC (3.1 mg, 5 mol% for Pd atom), and K2CO3 (18 mg, 0.13 mmol) in THF (0.2 mL) and H2O (0.2 mL) was stirred at 30 °C for 28 h. The resulting crude mixture was filtrated to recover PS-PdMC and washed with the same solvent and CHCl3/MeCN (1/1) in this order. The filtrate was added to water, and the aqueous phase was extracted with CHCl3 three times. The combined organic phase was washed with water, dried over anhydrous MgSO4, and concentrated under reduced pressure. The yields of 5, 6,2 and 72 were determined by 1H NMR using 1,1,2,2,-tetrachloroethane as an internal standard. The recovered PS-PdMC was dried under reduced pressure and applied to the same reaction condition.
References:
Yamamoto, Koji;Nameki, Riku;Sogawa, Hiromitsu;Takata, Toshikazu [Tetrahedron Letters,2020,vol. 61,# 21,art. no. 151870] Location in patent:supporting information

626-05-1
504 suppliers
$9.00/10g
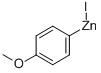
254454-47-2
19 suppliers
$162.00/50ml

193344-39-7
9 suppliers
inquiry

696-62-8
350 suppliers
$5.00/1g
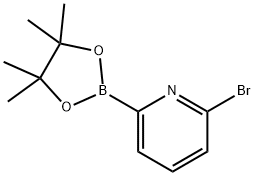
651358-83-7
155 suppliers
$65.00/500mg

193344-39-7
9 suppliers
inquiry

5720-07-0
464 suppliers
$6.00/5g
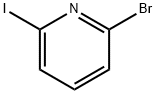
234111-08-1
149 suppliers
$17.00/250mg

193344-39-7
9 suppliers
inquiry