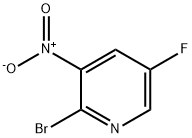
2-Bromo-5-fluoro-3-nitropyridine synthesis
- Product Name:2-Bromo-5-fluoro-3-nitropyridine
- CAS Number:652160-72-0
- Molecular formula:C5H2BrFN2O2
- Molecular Weight:220.98
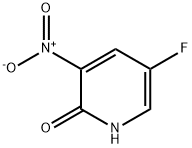
136888-20-5
143 suppliers
$8.00/100mg

652160-72-0
102 suppliers
$15.00/250mg
Yield: 77%
Reaction Conditions:
with N,N-dimethyl-formamide;phosphorus(V) oxybromide at 20 - 110; for 3.08333 h;
Steps:
A total of 117 g of 5-80 was divided in 4 batches of 30 g×3 and 27 g×1 and treated with POBr3 (3 equiv.; 163 g×3 and 155 g×1) and a catalytic amount of DMF (15 ml) at rt (DMF was added carefully gas evolution). After 5 min. at room temperature, the solutions were heated at 110° C. for 3 hr. LC/MS showed starting material had been consumed. The reaction mixtures were allowed to cool to rt. The reaction flasks were placed in an ice bath; and then ice was added very slowly and carefully portionwise into the flask, gas evolution was due to HBr formation; the liquid and black solid that formed was poured into a beaker with ice. EtOAc was added and the mixture was then extracted several times with EtOAc. The organic layer was washed with saturated aq. NaHCO3; H2O and brine; dried over Na2SO4 and filtered. The product was dried in the pump overnight to provide 123 g of 6-80 as a brown solid (77% yield). [1498] Note: Reaction is completed within 1 h. [1499] 1HNMR(δ, CDCl3):8.52 (m, 1H), 7.93 (m, 1H).
References:
Wang, Tao;Zhang, Zhongxing;Meanwell, Nicholas A.;Kadow, John F.;Yin, Zhiwei;Xue, Qiufen May;Regueiro-Ren, Alicia;Matiskella, John D.;Ueda, Yasutsugu US2004/110785, 2004, A1 Location in patent:Page 212
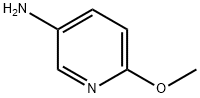
6628-77-9
350 suppliers
$8.00/5g

652160-72-0
102 suppliers
$15.00/250mg
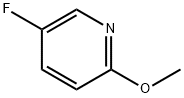
51173-04-7
235 suppliers
$8.00/1g

652160-72-0
102 suppliers
$15.00/250mg
51173-05-8
205 suppliers
$6.00/250mg

652160-72-0
102 suppliers
$15.00/250mg