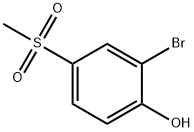
2-BROMO-4-(METHYLSULFONYL)PHENOL synthesis
- Product Name:2-BROMO-4-(METHYLSULFONYL)PHENOL
- CAS Number:20951-43-3
- Molecular formula:C7H7BrO3S
- Molecular Weight:251.1
14763-60-1
161 suppliers
$11.00/5g

20951-43-3
13 suppliers
inquiry
Yield:20951-43-3 85%
Reaction Conditions:
Stage #1:4-methanesulfonyl-phenol with trifluorormethanesulfonic acid in acetonitrile at -25 - -10;
Stage #2: with N-Bromosuccinimide in acetonitrile at -25 - 25;Solvent;Temperature;Reagent/catalyst;
Steps:
1.1.1; 3
Charge 4 (lOg, 58mmol) and acetonitrile (lOOmL) to a reaction vessel and start the stirrer. Adjust the batch to -18 °C to -22 °C (target -20 °C). Charge triflic acid (5.5mL, 62mmol) to the batch maintaining -10 °C to -25 °C (target -20 °C). Stir the batch at -10 °C to -25 °C (target -20 °C) for 10 to 20 minutes. Charge NBS (11.38g, 64mmol) to the batch at -10 °C to -25 °C (target -20 °C) and stir for ca. 30 min at -10 °C to -25 °C (target -20 °C). Warm the batch to 20 °C over 3-4 hours (reaction will occur when internal temp is between 5 °C and 15 °C). Stir the batch at 15 °C to 25 °C (target 20 °C) for approximately 1 hour and sample for reaction completion. (0520) [0269] If Compound 4 relative to Compound 5 is more than 5%: [0270] Cool the bath to -5 °C to -15 °C (target -10 °C) (cooling below 0 °C to ensure selectivity). Charge NBS to the batch according to the follow formula: Mass of NBS = (% Compound 4 x lOg). Warm the batch to 20 °C over 1-2 hours. Stir the batch at 15 °C to 25 °C (target 20 °C) for approximately 1 hour and check reaction for completion. Proceed to next line. (0521) [0271] If Compound 4 relative to Compound 5 is less than 5%: (0522) [0272] Warm the batch to 40 °C to 50 °C (target 48 °C). Concentrate the batch under reduced pressure to a final volume of ~40mL. Cool the batch to -15 °C to -5 °C (target -10 °C) and stir for ca. lh. Filter the batch by suction filtration. Slurry wash the filter cake with purified water (3 x 20mL) at 15 °C to 25 °C (target 20 °C) for 10 to 15 minutes each wash. Remove a sample of the filter cake for analysis by NMR. Continue washing cake until the residual succimide is below 1.0%mol% relative to 5. Dry the filter cake at up to 60°C under vacuum and nitrogen purge. Analyse the 5 by HPLC analysis (97%w/w to 99%w/w). Expected yield: 60-85% theory (90-110% w/w).
References:
CELGENE QUANTICEL RESEARCH, INC.;TRAVERSE, John Fitzgerald;YONG, Kelvin Hin-Yeong;FERRETTI, Antonio Christian;ALITE, Hekla;MOSELEY, Jonathan;RUDA, Antonio Maria;PRIMER, David;PHILP, Steven WO2020/23438, 2020, A2 Location in patent:Paragraph 0217-0218; 0224-0225; 0027-0228; 0267-0272