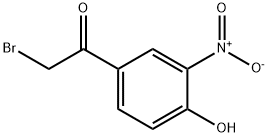
2-BROMO-4'-HYDROXY-3'-NITROACETOPHENONE synthesis
- Product Name:2-BROMO-4'-HYDROXY-3'-NITROACETOPHENONE
- CAS Number:5029-61-8
- Molecular formula:C8H6BrNO4
- Molecular Weight:260.04
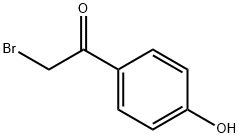
2491-38-5
268 suppliers
$5.00/100mg

5029-61-8
25 suppliers
$201.00/1g
Yield:5029-61-8 100%
Reaction Conditions:
with nitric acid in methanol at 0 - 20; for 3 h;Large scale;
Steps:
S1
S1: 2.13kg of raw material 2-bromo-4'-hydroxyacetophenone (1 equivalent), its structural formula is:Dissolve in 10L (5 volumes) methanol, and cool the resulting solution A to 0 ° C,And add 0.63kg of nitric acid dropwise to solution A,After the dropwise addition is completed, the resulting reaction system is reacted at room temperature for 3 hours,After the reaction is completed, the material is discharged under ice water conditions.Centrifuge the product obtained from the discharge, use the centrifugal force generated by the centrifuge to separate the liquid and solid particles in the product obtained from the discharge, and dry the solid particles2.59 kg of intermediate I was obtained. Intermediate I was a nitro compound with the structural formula:In this step, intermediate I is obtained by dissolving 2-bromo-4'-hydroxyacetophenone as a raw material in concentrated sulfuric acid and adding nitric acid, and the reaction yield is 100%.
References:
CN111018902,2020,A Location in patent:Paragraph 0014
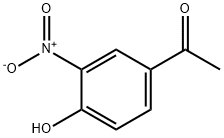
6322-56-1
272 suppliers
$9.00/10g

5029-61-8
25 suppliers
$201.00/1g