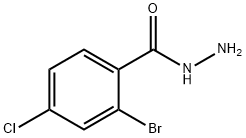
2-BROMO-4-CHLOROBENZHYDRAZIDE synthesis
- Product Name:2-BROMO-4-CHLOROBENZHYDRAZIDE
- CAS Number:1023334-50-0
- Molecular formula:C7H6BrClN2O
- Molecular Weight:249.49
936-08-3
238 suppliers
$11.00/5g

1023334-50-0
12 suppliers
inquiry
Yield:1023334-50-0 82%
Reaction Conditions:
Stage #1: 2-bromo-4-chlorobenzoic acidwith dmap;1,1'-carbonyldiimidazole in tetrahydrofuran at 70; for 3 h;Inert atmosphere;
Stage #2: with hydrazine in tetrahydrofuran at 20; for 1.75 h;
Steps:
2.7A 2-Bromo-4-chlorobenzohydrazide
Under argon, 1.50 g (6.18 mmol) of 2-bromo-4-chlorobenzoic acid were initially charged in 58.2 ml of tetrahydrofuran, 1.50 g (9.27 mmol) of 1,1 ‘-carbonyldiimidazole and 0.38 g (3.09 mmol) of 4-dimethylaminopyridine were added and the mixture was stirred at 70°C for 3 hours. The reaction was subsequently cooled to RT, and 8.03 ml (8.03 mmol) of hydrazine solution (1M in tetrahydrofuran) were then added in one portion. The mixture was 75 mm, and a further 8.03 ml of hydrazine solution were then added. After a further 30 mm with stirring, 60 ml of dichloromethane and 60 ml of saturated aqueous sodium bicarbonate solution were added. The organic phase wasremoved and the aqueous phase was extracted twice with dichloromethane. The combined organic phases were washed with water, dried over magnesium sulphate and concentrated under reduced pressure. The residue was purified by flash normal phase chromatography (silica gel, dichloromethane/methanol gradient), Yield: 1.30 g (82% of theory).LC/MS [Method 11]: R = 1.11 mm; MS (ESIpos): m/z = 249 (M+H),‘H-NMR (400 MHz, DMSO-d6): ? [ppm] = 9.58 (br. s., 1H), 7.81 (d, 1H), 7.52 (dd, 1H), 7.37 (d,1H), 4.49 (br. s., 2H).
References:
WO2017/5725,2017,A1 Location in patent:Page/Page column 83-84
57381-62-1
116 suppliers
$7.00/1g

1023334-50-0
12 suppliers
inquiry
690260-90-3
46 suppliers
$65.00/500mg

1023334-50-0
12 suppliers
inquiry