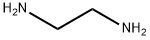2-Aminoethyl(ethyl)amine synthesis
- Product Name:2-Aminoethyl(ethyl)amine
- CAS Number:110-72-5
- Molecular formula:C4H12N2
- Molecular Weight:88.15
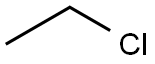
75-00-3
219 suppliers
$29.39/crm40015

110-72-5
273 suppliers
$14.00/10g
Yield:-
Reaction Conditions:
with ethylenediamine in n-heptane
Steps:
7 EXAMPLE 7
EXAMPLE 7 To a glass-lined reactor is charged 848 parts of ethylenediamine (98%) followed by 331 parts of liquid ethyl chloride, charged through a dip leg at a rate to maintain the reaction mixture at 45°-55° C. The mixture is stirred for 2 hours and 91 parts of heptane are added thereto. The excess ethylenediamine is azeotropically distilled off through a packed column with recycle of the heptane distillate to the top of the column. A total of 492 parts of ethylenediamine is recovered from the distillate for recycle. The reaction mixture is neutralized with 402 parts of 50% caustic soda, cooled to room temperature, and centrifuged to remove the sodium chloride by-product. The salt cake is washed with 70 parts of heptane and the wash liquor is combined with the mother liquor in a glass-lined vessel. Water is azeotropically distilled off from the combined liquors through a packed column with recycle of the distilled heptane to the top of the column. After all of the water is removed the heptane is fractionally distilled off through the packed column to obtain a residue containing 273 parts of N-ethylethylenediamine. This residue is reserved for subsequent combination with the residues of Examples 8-10.
References:
American Cyanamid Company US4217308, 1980, A
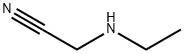
24426-40-2
10 suppliers
inquiry

110-72-5
273 suppliers
$14.00/10g

24426-40-2
10 suppliers
inquiry

110-72-5
273 suppliers
$14.00/10g
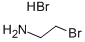
2576-47-8
482 suppliers
$8.00/25g
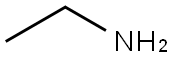
75-04-7
396 suppliers
$10.00/20mg

110-72-5
273 suppliers
$14.00/10g