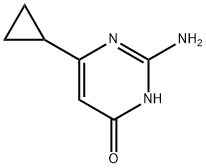
2-amino-6-cyclopropylpyrimidin-4-ol synthesis
- Product Name:2-amino-6-cyclopropylpyrimidin-4-ol
- CAS Number:21573-08-0
- Molecular formula:C7H9N3O
- Molecular Weight:151.17
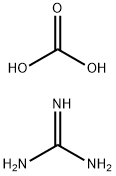
593-85-1
494 suppliers
$6.00/25g
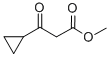
32249-35-7
214 suppliers
$5.00/5g

21573-08-0
17 suppliers
$67.00/50mg
Yield:21573-08-0 7.2 g
Reaction Conditions:
with sodium ethanolate in ethanol;Reflux;
Steps:
1 Step 1: 2-amino-6-cydopropylpyrimidin-4-ol
To a suspension of methyl 3 -cyclopropyl-3 -oxopropanoate (8.1 g, 57 mmol) and guanidine carbonate (5.65 g, 63 mmol) in ethanol (70 mL) at rt, was added NaOEt (7.75 g, 114 mmol) portion-wise over 20 min. After the addition, the resulting mixture was warmed to reflux and stirred overnight. After the reaction was complete as indicated by TLC analysis, the resulting suspension was concentrated under reduced pressure to remove most of the ethanol. The residue was diluted with a saturated NELCl solution (100 mL) and extracted with EA (3 x 200 mL). The combined organic layer was dried with Na?.S04 (50 g), filtered and concentrated to give 9 g of crude product. The crude product was slurried in a mixed solvent (Methanol/MTBE = 1/10, 33 mL) at rt overnight. After filtration and drying, 7.2 g of the title compound was obtained. LC-MS (Method A) (ESI+): m/z 152 (M+H)+; 1H-NMR (300 MHz, DMSO -d6) d 10.45 (s, 1H), 6.37 (s, 2H), 5.48 (s, 1H), 1.65 (m, 1H), 078-0.83 (m, 4H).
References:
WO2020/132269,2020,A1 Location in patent:Paragraph 0664

32249-35-7
214 suppliers
$5.00/5g
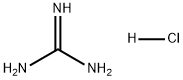
50-01-1
766 suppliers
$5.00/25g

21573-08-0
17 suppliers
$67.00/50mg