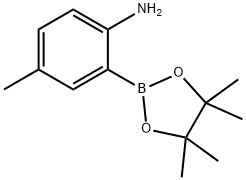
2-AMINO-5-METHYLPHENYBORONIC ACID, PINACOL ESTER synthesis
- Product Name:2-AMINO-5-METHYLPHENYBORONIC ACID, PINACOL ESTER
- CAS Number:948592-80-1
- Molecular formula:C13H20BNO2
- Molecular Weight:233.11
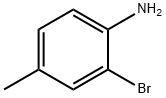
583-68-6
292 suppliers
$10.00/5G
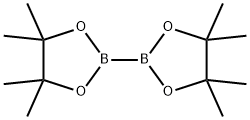
73183-34-3
581 suppliers
$6.00/5g

948592-80-1
51 suppliers
$60.00/100mg
Yield:948592-80-1 94%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in dimethyl sulfoxide at 80;Inert atmosphere;
Steps:
119A 11 9A. Preparation of 4-methyl-2-(4,4,5 ,5 -tetramethyl- 1,3 ,2-dioxaborolan-2-yl)aniline
2-Bromo-4-methylaniline (3 g, 16.12 mmol), bis(pinacolato)diboron (6.14 g,24.19 mmol), KOAc (4.07 g, 41.4 mmol) were added to DMSO (9 mL) under N2 atm and then degassed for 10 mm. Pd(dppf)C12.CH2Cl2Adduct (0.395 g, 0.484 mmol) was added and the resulting suspension was stirred overnight at 80 °C. The reaction mixture was partitioned between DCM and water. The organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated then purified normal phase chromatography usinghexane and EtOAc as eluents to give 4-methyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (3.52 g, 94% yield) as a clear oil which solidified into a white solid upon standing. MS(ESI) m/z: 152.3 (M-C6H,o+H). ‘H NMR (400MHz, CDC13-d) ? 7.43 (d, J=1.8 Hz, 1H), 7.04 (dd, J=8.3, 2.3 Hz, 1H), 6.55 (d, J=8.1 Hz, 1H), 4.60 (br. s., 2H), 2.23- 2.20 (m, 3H), 1.38 - 1.32 (m, 12H).
References:
WO2015/116886,2015,A1 Location in patent:Page/Page column 351

583-68-6
292 suppliers
$10.00/5G
25015-63-8
221 suppliers
$22.39/5G

948592-80-1
51 suppliers
$60.00/100mg
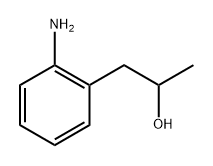
65826-91-7
2 suppliers
inquiry

25015-63-8
221 suppliers
$22.39/5G

948592-80-1
51 suppliers
$60.00/100mg