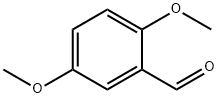
2,5-Dimethoxybenzaldehyde synthesis
- Product Name:2,5-Dimethoxybenzaldehyde
- CAS Number:93-02-7
- Molecular formula:C9H10O3
- Molecular Weight:166.17
Reaction
A : Anisaldehyde from anethole via oxidative cleavage: 20 g anise oil was suspended in a mixture of 150 mL water and 30 mL conc. sulfuric acid; addition of 55 g sodium bichromate at such a rate that the temperature did not exceed 40°C. The reaction mixture was extracted with 4 x 125 mL toluene and the solvent evaporated. The residual oil was vacuum distilled to yield 9.1 g anisaldehyde.
B : O-formyl-4-methoxyphenol: 6 mL anisaldehyde was dissolved in 75 mL dichloromethane (DCM). A mixture of 12 g hydrogen peroxide and 10 mL conc. formic acid was added over 30 min. The reaction mixture was gently refluxed for 21 h.
C: B 4-methoxyphenol: Evaporating the solvent from reaction mixture and taking up the residue in 100 mL aqueous NaOH (20%) (25 mL MeOH as co-solvent) yielded 4.1 g 4-methoxyphenol as a white crystalline product after the usual work-up and purification steps.
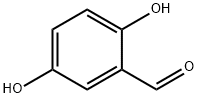
D : Reimer-Tiemann formylation of 4-methoxyphenol: 124.1 g 4-methoxyphenol was dissolved in NaOH solution (320 g NaOH in 400 mL water). In total, 161 mL chloroform was added. The usual work-up and steam distillation yielded 109.8 g of a clear yellow oil that did not solidify upon standing at room temperature (GC/MS: 94% 2-hydroxy-5-methoxybenzaldehyde).
E: D Methylation of 2-hydroxy-5-methoxybenzaldehyde: The yellow oil from was used without further purification. A 250 mL RB flask was charged with 100 mL acetone, 14 g anhydrous potassium carbonate and 10 g 2-hydroxy-5-methoxybenzaldehyde; the mixture was brought at reflux temperature and 11 g dimethyl sulfate was added. The reaction was continued for 4 hours. The solvent is evaporated and the crude end product crystallized in cold water. Recrystallization from EtOH/water yielded 8.3 g 2,5-dimethoxybenzaldehyde (GC/MS: 98%+ 2,5-dimethoxybenzaldehyde)
Yield:93-02-7 97.6%
Reaction Conditions:
with titanium tetrachloride in dichloromethane at -5 - 20; for 1.5 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
1.A-9.A Example 2
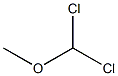
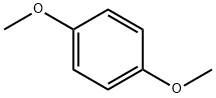
A. Add 13.82g (0.1mol) of 1,4-dimethoxybenzene in a 500mL three-necked round bottom flask, 200mL of anhydrous dichloromethane, nitrogen protection, and cool down to -5°C. Add titanium tetrachloride (13mL) , 0.12mol) of anhydrous dichloromethane solution 45mL, add dropwise 90mL of 1,1-dichloromethyl ether (14.94g, 0.13mol) in anhydrous dichloromethane solution, add dropwise after 1h, stir at room temperature and react for 30min Add 300g ice cubes to the reaction solution, 8mL concentrated hydrochloric acid, stir for 2h, separate the liquids, extract the aqueous phase with dichloromethane twice, 100mL each time, combine the organic phases, wash the organic phase twice with water, 100mL each time, anhydrous Dry over sodium sulfate and concentrate under reduced pressure to obtain 16.22 g of oily 2,5-dimethoxybenzaldehyde, with a yield of 97.6% and HPLC 97.9%
References:
CN112321413,2021,A Location in patent:Paragraph 0024-0026; 0032-0034; 0040-0042; 0047-0049; 0055
33524-31-1
101 suppliers
$19.00/1g

93-02-7
571 suppliers
$6.00/5g
![1,3-Dioxane-4,6-dione, 5-[(2,5-dimethoxyphenyl)methylene]-2,2-dimethyl-](/CAS/20210111/GIF/117646-10-3.gif)
117646-10-3
0 suppliers
inquiry

93-02-7
571 suppliers
$6.00/5g
![Propanedinitrile, [(2,5-dimethoxyphenyl)methylene]-](/CAS/20180601/GIF/2972-75-0.gif)
2972-75-0
5 suppliers
inquiry

93-02-7
571 suppliers
$6.00/5g