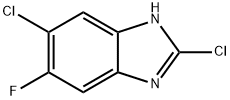
2,5-DICHLORO-6-FLUOROBENZIMIDAZOLE synthesis
- Product Name:2,5-DICHLORO-6-FLUOROBENZIMIDAZOLE
- CAS Number:142356-65-8
- Molecular formula:C7H3Cl2FN2
- Molecular Weight:205.02
![5-Chloro-6-Fluoro-1H-Benzo[D]IMidazol-2(3H)-One](/CAS/GIF/1075753-25-1.gif)
1075753-25-1
22 suppliers
$195.00/1g

142356-65-8
21 suppliers
$60.00/50mg
Yield:142356-65-8 77%
Reaction Conditions:
with trichlorophosphate at 120; for 2 h;
Steps:
94.b
(b) Preparation of the intermediate compounds 2,5-dichloro-6-fluoro-lH-benzo[d]imidazole and 2,6-dichloro-5-fluoro-lH-benzo[d]imidazole.To 5-chloro-6-fluoro-lH-benzo[d]imidazol-2(3Η)-one (1 g, 5.3 mmol), phosphoryl trichloride (POCI3, 132 mmol)) was added, the reaction mixture was heated at 12O0C for 2 hours and then concentrated in vacuo. The residue was neutralized with sodium hydroxide, the resulting was filtered off, washed with water and dried to give 0.85 g (77 % yield) of 2,5-dichloro-6-fluoro- lH-benzo[d]imidazole and 2,6-dichloro-5-fluoro-lH-benzo[d]imidazole after recrystallization after petroleum ether. No effort was made to determine the ratios of the tautomers.
References:
WO2011/23812,2011,A1 Location in patent:Page/Page column 120-121
139512-70-2
118 suppliers
$9.00/250mg

142356-65-8
21 suppliers
$60.00/50mg